Abstract
AIM—Human papilloma virus (HPV) types 16 and 18 have been associated with neoplastic conditions of the conjunctiva. However, the presence of this virus has not been reported in non-neoplastic disorders of the external eye nor has it been studied in normal conjunctival tissues. METHODS—Ninety six paraffin embedded tissue specimens with neoplastic and non-neoplastic lesions and 19 conjunctiva samples free from overt disease were studied for HPV types 16 and 18 positivity with the polymerase chain reaction. RESULTS—HPV types 16 and 18 DNA were identified in 57% of in situ squamous cell carcinoma, in 55% of invasive squamous cell carcinoma, in 20% of climatic droplet keratopathy, in 35% of scarred corneas, and in 32% of normal conjunctival tissue obtained during routine cataract extractions. CONCLUSION—These findings indicate that HPV types 16 and 18 are detectable with the polymerase chain reaction not only in epithelial neoplasms of the ocular mucous membrane but also in non-neoplastic lesions as well as in apparently healthy conjunctiva.
Full Text
The Full Text of this article is available as a PDF (133.2 KB).
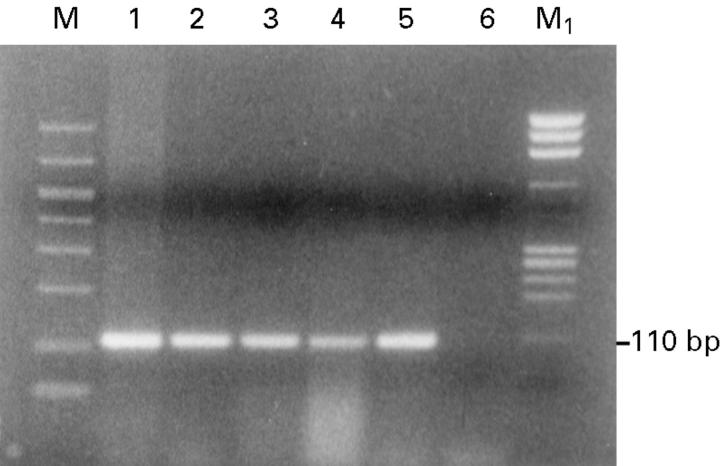
Figure 1 .
Detection of β globin gene. Ethidium bromide stained 2% agarose gel showing β globin gene amplification (110 bp product) following PCR amplification using PCO3/PCO4 primer and 1 µg of DNA as a template from the following samples: lane 1, positive control (normal blood lymphocytes); lane 2, invasive squamous cell carcinoma (SCC); lane 3, in situ SCC; lane 4, corneal scar; lane 5, conjunctiva; lane 6, negative control (no DNA); M, GelMarker; M1, PhiX 174 RF Hae III marker.
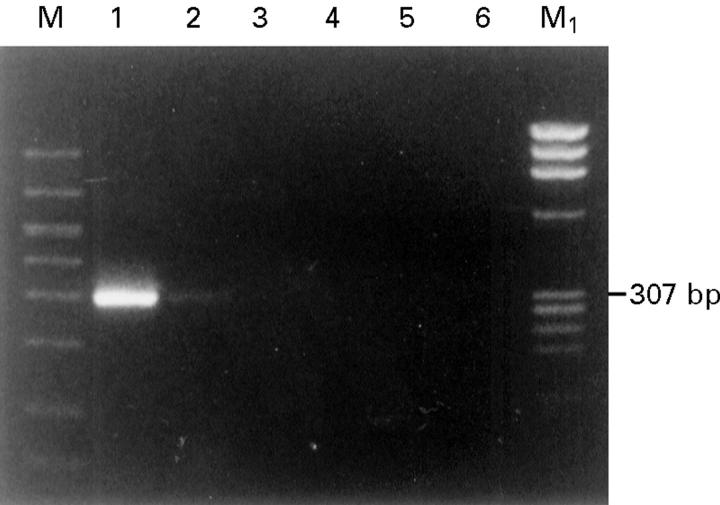
Figure 2 .
First PCR amplification of HPV DNA: consensus primer for E6 ORF of HPV 16 and 18 was used as outer primer along with 1 µg of DNA as a template (307 bp product) from the following samples: lane 1, positive control for HPV 16 (CaSki cell DNA); lane 2, positive control for HPV 18 (HeLa cell DNA); lane 3, conjunctiva; lane 4, invasive squamous cell carcinoma (SCC); lane 5, in situ SCC; lane 6, negative control (no DNA); M, GelMarker; M1, PhiX 174 RF Hae III marker.
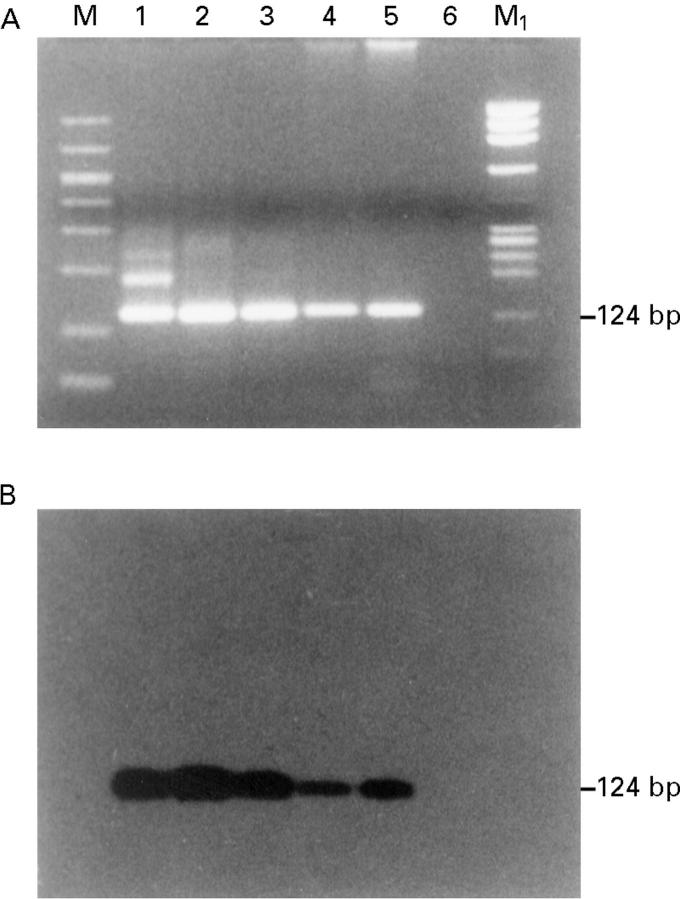
Figure 3 .
Detection of HPV 16 DNA. HPV 16 specific inner primer was used along with 2 µl of the first PCR reaction mixture as a template. (A) Analysis of the amplification DNA products on 2% agarose gel stained with ethidium bromide (124 bp product). (B) Southern blot analysis of the amplification DNA products hybridised with non-radioactive labelled HPV 16 specific oligonucleotide probe for the following samples: lane 1, positive control (CaSki cell DNA); lane 2, conjunctiva; lane 3, conjunctiva; lane 4, invasive squamous cell carcinoma (SCC); lane 5, in situ SCC; lane 6, negative control (no DNA); M, GelMarker; M1, PhiX 174 RF Hae III marker.
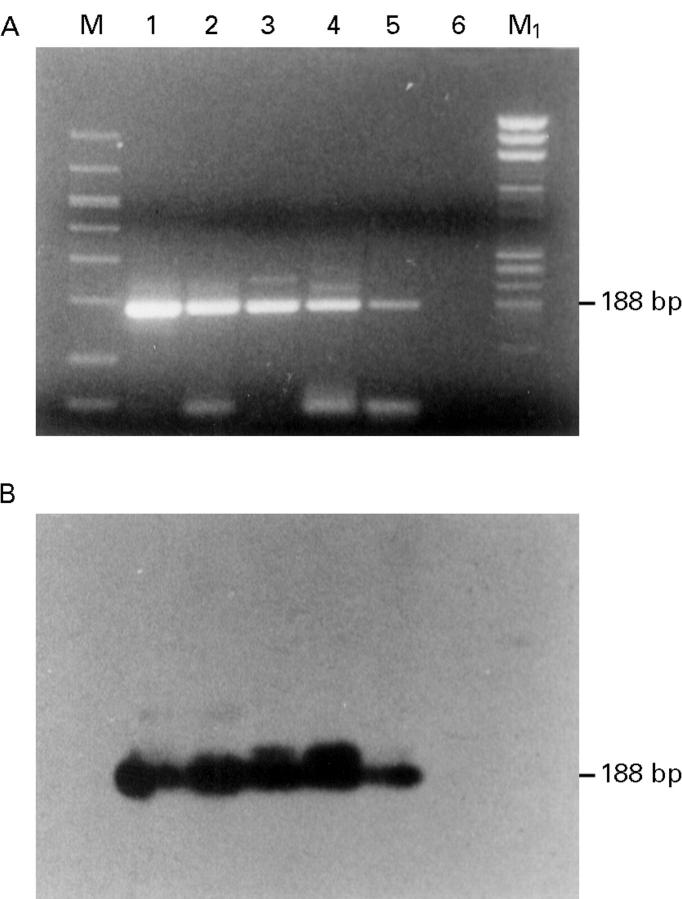
Figure 4 .
Detection of HPV 18 DNA. HPV 18 specific inner primer was used along with 2 µl of the first PCR reaction mixture as a template. (A) Analysis of the amplification DNA products on 2% agarose gel stained with ethidium bromide (188 bp product). (B) Southern blot of the amplification products hybridised with non-radioactive labelled HPV 18 specific oligonucleotide probe for the following samples: lane 1, positive control for HPV 16 (HeLa cell DNA); lane 2, conjunctiva; lane 3, invasive squamous cell carcinoma (SCC); lane 4, invasive SCC; lane 5, in situ SCC; lane 6, negative control (no DNA); M, GelMarker; M1, PhiX 174 RF Hae III marker.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Wyllie A. H., Bird C. C. Papillomaviruses and human cancer. Hum Pathol. 1990 Jul;21(7):686–698. doi: 10.1016/0046-8177(90)90027-3. [DOI] [PubMed] [Google Scholar]
- Chan M. K., Lau K. M., Tsui Y., Wong F. W., Huang D. P. Human papillomavirus infection in Hong Kong Chinese women with normal and abnormal cervix--detection by polymerase chain reaction method on cervical scrapes. Gynecol Oncol. 1996 Feb;60(2):217–223. doi: 10.1006/gyno.1996.0028. [DOI] [PubMed] [Google Scholar]
- Howley P. M. The role of papillomaviruses in human cancer. Important Adv Oncol. 1987:55–73. [PubMed] [Google Scholar]
- Jalal H., Sanders C. M., Prime S. S., Scully C., Maitland N. J. Detection of human papilloma virus type 16 DNA in oral squames from normal young adults. J Oral Pathol Med. 1992 Nov;21(10):465–470. doi: 10.1111/j.1600-0714.1992.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. J. Aetiology of spheroidal degeneration of the cornea in Labrador. Br J Ophthalmol. 1981 Apr;65(4):270–283. doi: 10.1136/bjo.65.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. A., Hirst L. W. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995 May-Jun;39(6):429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- Lee G. A., Williams G., Hirst L. W., Green A. C. Risk factors in the development of ocular surface epithelial dysplasia. Ophthalmology. 1994 Feb;101(2):360–364. doi: 10.1016/s0161-6420(94)31328-5. [DOI] [PubMed] [Google Scholar]
- Levine A. J. Oncogenes of DNA tumor viruses. Cancer Res. 1988 Feb 1;48(3):493–496. [PubMed] [Google Scholar]
- Maitland N. J., Cox M. F., Lynas C., Prime S. S., Meanwell C. A., Scully C. Detection of human papillomavirus DNA in biopsies of human oral tissue. Br J Cancer. 1987 Sep;56(3):245–250. doi: 10.1038/bjc.1987.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J. M., Mayr A. J., Martin W. J. DNA of human papillomavirus type 16 in dysplastic and malignant lesions of the conjunctiva and cornea. N Engl J Med. 1989 Jun 1;320(22):1442–1446. doi: 10.1056/NEJM198906013202202. [DOI] [PubMed] [Google Scholar]
- McDonnell J. M., McDonnell P. J., Sun Y. Y. Human papillomavirus DNA in tissues and ocular surface swabs of patients with conjunctival epithelial neoplasia. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):184–189. [PubMed] [Google Scholar]
- Nawa A., Nishiyama Y., Kikkawa F., Kawai M., Mano H., Goto S., Suganuma N., Tomoda Y., Nakashima N. Detection of human papillomaviruses from histologically normal lymph nodes of Japanese cervical cancer patients by nested polymerase chain-reaction assay. Int J Cancer. 1993 Apr 1;53(6):932–937. doi: 10.1002/ijc.2910530611. [DOI] [PubMed] [Google Scholar]
- Parashari A., Singh V., Gupta M. M., Satyanarayana L., Chattopadhya D., Sodhani P., Sehgal A. Significance of inflammatory cervical smears. APMIS. 1995 Apr;103(4):273–278. doi: 10.1111/j.1699-0463.1995.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Pfister H., Gassenmaier A., Nürnberger F., Stüttgen G. Human papilloma virus 5-DNA in a carcinoma of an epidermodysplasia verruciformis patient infected with various human papillomavirus types. Cancer Res. 1983 Mar;43(3):1436–1441. [PubMed] [Google Scholar]
- Rotkin I. D. A comparison review of key epidemiological studies in cervical cancer related to current searches for transmissible agents. Cancer Res. 1973 Jun;33(6):1353–1367. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Taylor H. R. Aetiology of climatic droplet keratopathy and pterygium. Br J Ophthalmol. 1980 Mar;64(3):154–163. doi: 10.1136/bjo.64.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress G., Kónya J., Csiky-Mészáros T., Czeglédy J., Gergely L. Human papillomavirus DNA and anti-HPV secretory IgA antibodies in cytologically normal cervical specimens. J Med Virol. 1994 Jun;43(2):201–207. doi: 10.1002/jmv.1890430219. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1–30. doi: 10.1007/978-3-642-66800-5_1. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in human cancer. Cancer. 1987 May 15;59(10):1692–1696. doi: 10.1002/1097-0142(19870515)59:10<1692::aid-cncr2820591003>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]