Full Text
The Full Text of this article is available as a PDF (174.7 KB).
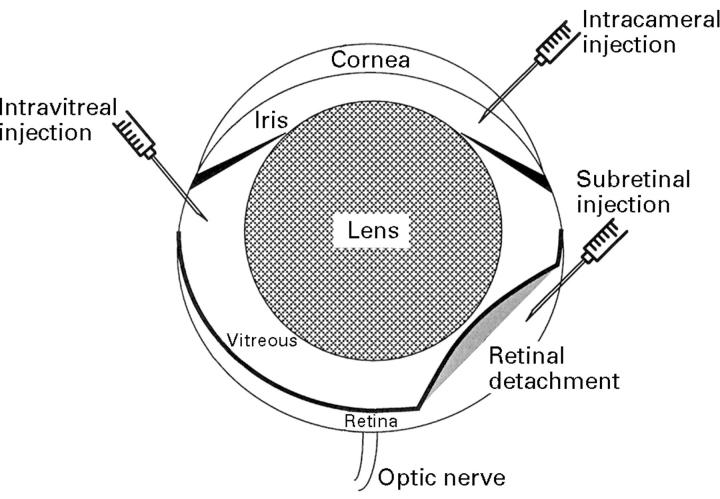
Figure 1 .
Schematic of the mouse eye. Note that the lens is proportionally much larger in mice than in humans. The routes of intraocular injections are indicated.
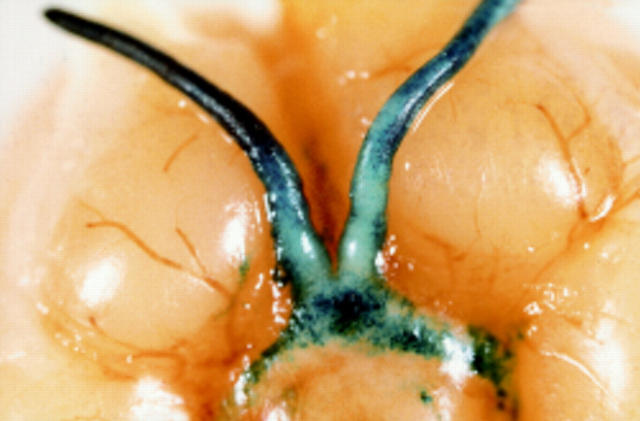
Figure 2 .
Blue X-gal staining indicates lacZ activity in the optic nerve and optic chiasma of a BALB/c mouse 3 days after subretinal injection of 2 µl suspension of replication competent HSV virus, BE8 (5 × 109 pfu/ml) in which the lacZ, driven by a CMV promoter, has been inserted into the non-essential Us5 gene.57
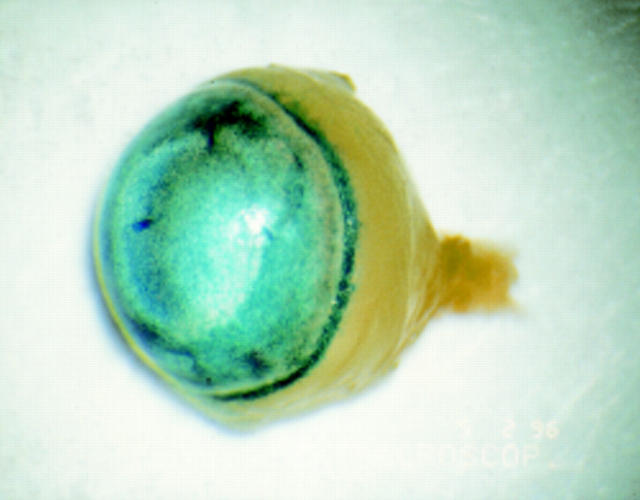
Figure 3 .
Blue X-gal staining in the anterior chamber of adult BALB/c mouse after intracameral injection of 2 µl of adenovirus carrying a lacZ gene with nuclear localisation signal driven by a CMV promoter (AV.CMV.LacZnuc) at a titre of 1 × 109 pfu/ml. LacZ activity can be observed throughout the anterior segment including corneal endothelium, iris, and trabecular meshwork.
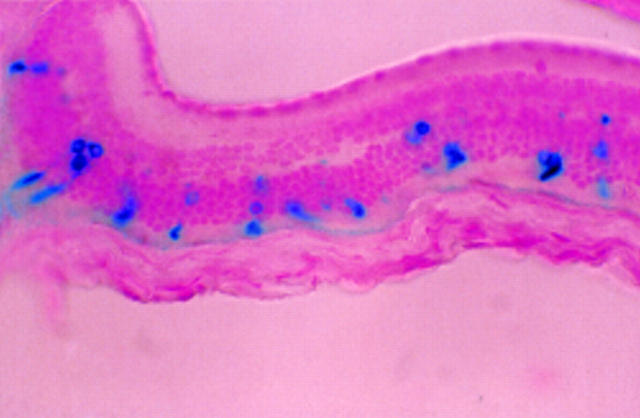
Figure 4 .
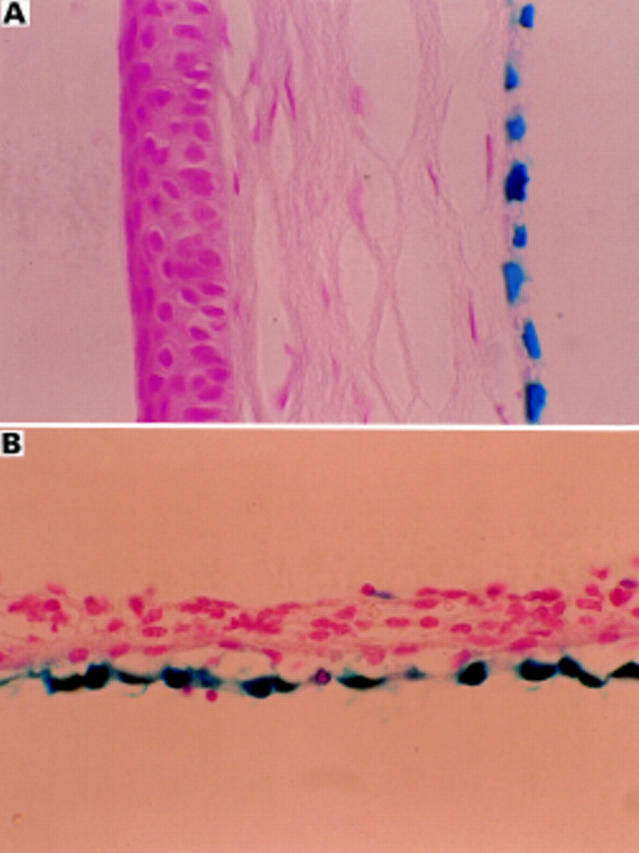
Transduced corneal endothelium (A) and iris pigment epithelium (B) in adult BALB/c mouse after intracameral injection of 2 µl of AV.CMV.LacZnuc (1 × 109 pfu/ml). A 5 µm paraffin section counterstained with nuclear fast red (× 45).
Figure 5 .
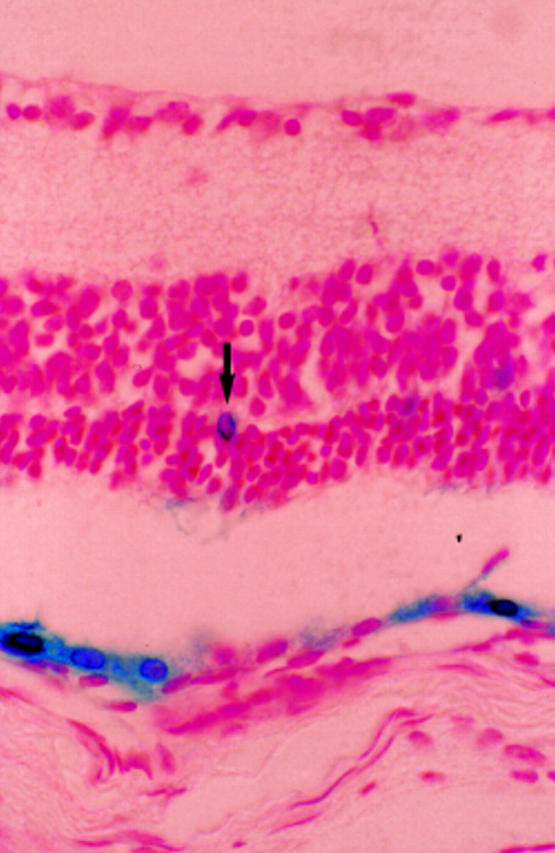
Transduced retinal pigment epithelium and occasional positive photoreceptor cell (arrow) in adult BALB/c mouse 2 weeks after subretinal injection of 2 µl of AV.CMV.LacZnuc (1 × 109 pfu/ml). A 5 µm paraffin section counterstained with nuclear fast red (× 66).
Figure 6 .
Transduced photoreceptor cells in adult nude mouse 1 month after subretinal injection of 2 µl of AAV.CMV.LacZ (1 × 107 IU/ml). All the stained outer segments can be related to stained photoreceptor nuclei which is consistent with transduction of photoreceptor cells and subsequent transport of LacZ into the outer segments. A 5 µm paraffin section counterstained with nuclear fast red (×25).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., da Silva J. L., Lavrovsky Y., Stoltz R. A., Kappas A., Dunn M. W., Schwartzman M. L. Adenovirus-mediated heme oxygenase-1 gene transfer into rabbit ocular tissues. Invest Ophthalmol Vis Sci. 1995 Oct;36(11):2202–2210. [PubMed] [Google Scholar]
- Ali M., Lemoine N. R., Ring C. J. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1994 Nov;1(6):367–384. [PubMed] [Google Scholar]
- Ali R. R., Reichel M. B., Thrasher A. J., Levinsky R. J., Kinnon C., Kanuga N., Hunt D. M., Bhattacharya S. S. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet. 1996 May;5(5):591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- Aramant R. B., Seiler M. J. Fiber and synaptic connections between embryonic retinal transplants and host retina. Exp Neurol. 1995 Jun;133(2):244–255. doi: 10.1006/exnr.1995.1027. [DOI] [PubMed] [Google Scholar]
- Balan P., Davis-Poynter N., Bell S., Atkinson H., Browne H., Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994 Jun;75(Pt 6):1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- Bascom R. A., Liu L., Heckenlively J. R., Stone E. M., McInnes R. R. Mutation analysis of the ROM1 gene in retinitis pigmentosa. Hum Mol Genet. 1995 Oct;4(10):1895–1902. doi: 10.1093/hmg/4.10.1895. [DOI] [PubMed] [Google Scholar]
- Bennett J., Tanabe T., Sun D., Zeng Y., Kjeldbye H., Gouras P., Maguire A. M. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996 Jun;2(6):649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- Bennett J., Wilson J., Sun D., Forbes B., Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2535–2542. [PubMed] [Google Scholar]
- Bird A. C. Retinal photoreceptor dystrophies LI. Edward Jackson Memorial Lecture. Am J Ophthalmol. 1995 May;119(5):543–562. doi: 10.1016/s0002-9394(14)70212-0. [DOI] [PubMed] [Google Scholar]
- Borrás T., Tamm E. R., Zigler J. S., Jr Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1282–1293. [PubMed] [Google Scholar]
- Budenz D. L., Bennett J., Alonso L., Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995 Oct;36(11):2211–2215. [PubMed] [Google Scholar]
- Chang B., Bronson R. T., Hawes N. L., Roderick T. H., Peng C., Hageman G. S., Heckenlively J. R. Retinal degeneration in motor neuron degeneration: a mouse model of ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):1071–1076. [PubMed] [Google Scholar]
- Chang G. Q., Hao Y., Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993 Oct;11(4):595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Culver K. W. Measuring success in clinical gene therapy research. Mol Med Today. 1996 Jun;2(6):234–236. doi: 10.1016/1357-4310(96)88803-4. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Finn J. T., Peng Y. W., McGee T. L., Berson E. L., Yau K. W. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Ferrari F. K., Samulski T., Shenk T., Samulski R. J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996 May;70(5):3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. J., Gao G. P., Weitzman M. D., DeMatteo R., Burda J. F., Wilson J. M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996 Jan;70(1):520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Du J., Kjeldbye H., Yamamoto S., Zack D. J. Long-term photoreceptor transplants in dystrophic and normal mouse retina. Invest Ophthalmol Vis Sci. 1994 Jul;35(8):3145–3153. [PubMed] [Google Scholar]
- Gouras P., Du J., Kjeldbye H., Yamamoto S., Zack D. J. Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest Ophthalmol Vis Sci. 1992 Aug;33(9):2579–2586. [PubMed] [Google Scholar]
- Hawkins R. K., Jansen H. G., Sanyal S. Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985 Dec;41(6):701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Hodgson C. P., Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol. 1996 Mar;14(3):339–342. doi: 10.1038/nbt0396-339. [DOI] [PubMed] [Google Scholar]
- Huang P. C., Gaitan A. E., Hao Y., Petters R. M., Wong F. Cellular interactions implicated in the mechanism of photoreceptor degeneration in transgenic mice expressing a mutant rhodopsin gene. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8484–8488. doi: 10.1073/pnas.90.18.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Tashiro F., Ikuta K., Asano T., Katagiri H., Inukai K., Kikuchi M., Yazaki Y., Oka Y., Miyazaki J. Inhibition of pancreatic beta-cell glucokinase by antisense RNA expression in transgenic mice: mouse strain-dependent alteration of glucose tolerance. FEBS Lett. 1995 Sep 11;371(3):329–332. doi: 10.1016/0014-5793(95)00932-y. [DOI] [PubMed] [Google Scholar]
- Jiang L. Q., Jorquera M., Streilein J. W. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3347–3354. [PubMed] [Google Scholar]
- Jomary C., Piper T. A., Dickson G., Couture L. A., Smith A. E., Neal M. J., Jones S. E. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994 Jun 27;347(2-3):117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Khillan J. S., Li S. W., Prockop D. J. Partial rescue of a lethal phenotype of fragile bones in transgenic mice with a chimeric antisense gene directed against a mutated collagen gene. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6298–6302. doi: 10.1073/pnas.91.14.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Sakamoto T., Cardillo J. A., Spee C., Hinton D. R., Gordon E. M., Anderson W. F., Ryan S. J. Retrovirus-mediated suicide gene transduction in the vitreous cavity of the eye: feasibility in prevention of proliferative vitreoretinopathy. Hum Gene Ther. 1996 May 1;7(7):799–808. doi: 10.1089/hum.1996.7.7-799. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Unoki K., Yasumura D., Matthes M. T., Yancopoulos G. D., Steinberg R. H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Adamian M., Roof D. J., Berson E. L., Dryja T. P., Roessler B. J., Davidson B. L. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2543–2549. [PubMed] [Google Scholar]
- Li T., Davidson B. L. Phenotype correction in retinal pigment epithelium in murine mucopolysaccharidosis VII by adenovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7700–7704. doi: 10.1073/pnas.92.17.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R. M. Neurotrophic growth factors and neurodegenerative diseases: therapeutic potential of the neurotrophins and ciliary neurotrophic factor. Neurobiol Aging. 1994 Mar-Apr;15(2):249–251. doi: 10.1016/0197-4580(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Little C. W., Castillo B., DiLoreto D. A., Cox C., Wyatt J., del Cerro C., del Cerro M. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci. 1996 Jan;37(1):204–211. [PubMed] [Google Scholar]
- Lokensgard J. R., Bloom D. C., Dobson A. T., Feldman L. T. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994 Nov;68(11):7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. E., Sandberg M. A., Berson E. L., Dryja T. P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993 Jun;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Meindl A., Dry K., Herrmann K., Manson F., Ciccodicola A., Edgar A., Carvalho M. R., Achatz H., Hellebrand H., Lennon A. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat Genet. 1996 May;13(1):35–42. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- Mullen R. J., LaVail M. M. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976 May 21;192(4241):799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Naash M. I., Hollyfield J. G., al-Ubaidi M. R., Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Olsson J. E., Gordon J. W., Pawlyk B. S., Roof D., Hayes A., Molday R. S., Mukai S., Cowley G. S., Berson E. L., Dryja T. P. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992 Nov;9(5):815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Cousin P., Aubert J. F., Brunner H. R. Transient inhibition of angiotensinogen production in transgenic mice bearing an antisense angiotensinogen gene. Kidney Int. 1995 Jun;47(6):1638–1646. doi: 10.1038/ki.1995.228. [DOI] [PubMed] [Google Scholar]
- Pepose J. S., Leib D. A. Herpes simplex viral vectors for therapeutic gene delivery to ocular tissues. Recent breakthroughs in the molecular genetics of ocular diseases. Invest Ophthalmol Vis Sci. 1994 May;35(6):2662–2666. [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Rosenfeld P. J., Cowley G. S., McGee T. L., Sandberg M. A., Berson E. L., Dryja T. P. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992 Jun;1(3):209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Sanyal S., De Ruiter A., Hawkins R. K. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980 Nov 1;194(1):193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Sheng Y., Gouras P., Cao H., Berglin L., Kjeldbye H., Lopez R., Rosskothen H. Patch transplants of human fetal retinal pigment epithelium in rabbit and monkey retina. Invest Ophthalmol Vis Sci. 1995 Feb;36(2):381–390. [PubMed] [Google Scholar]
- Silverman M. S., Hughes S. E., Valentino T. L., Liu Y. Photoreceptor transplantation: anatomic, electrophysiologic, and behavioral evidence for the functional reconstruction of retinas lacking photoreceptors. Exp Neurol. 1992 Jan;115(1):87–94. doi: 10.1016/0014-4886(92)90227-h. [DOI] [PubMed] [Google Scholar]
- Unoki K., LaVail M. M. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):907–915. [PubMed] [Google Scholar]
- Yamamoto S., Du J., Gouras P., Kjeldbye H. Retinal pigment epithelial transplants and retinal function in RCS rats. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3068–3075. [PubMed] [Google Scholar]
- Zolotukhin S., Potter M., Hauswirth W. W., Guy J., Muzyczka N. A "humanized" green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996 Jul;70(7):4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker C. L., Ehinger B., Seiler M., Aramant R. B., Adolph A. R. Ultrastructural circuitry in retinal cell transplants to rat retina. J Neural Transplant Plast. 1994 Jan-Mar;5(1):17–29. doi: 10.1155/NP.1994.17. [DOI] [PMC free article] [PubMed] [Google Scholar]