Abstract
AIM—To present new morphological observations of intraepithelial capillaries in pterygium and to provide some explanations for this phenomenon. METHODS—The ultrastructural features of pterygia from 26 patients were examined. Surgically excised tissue was processed for conventional light and transmission electron microscopy. RESULTS—Individual capillaries within the epithelium of the anterior half towards the head of pterygia were identified in 11 specimens out of 26 pterygia examined (42.3%). The perivascular connective tissue of the intraepithelial capillaries contained fibroblasts, collagen fibrils, and elastin-like material. Epithelial cells surrounding these capillaries showed defects in the basal lamina in contrast with the continuous basal lamina of the endothelium. In the intercellular space of the epithelium an amorphous substance, occasional fibroblast processes, and collagen fibrils were frequently observed. CONCLUSION—Capillaries in the epithelium of pterygia are rare, but not exceptional. The ingrowth of these vessels from the stroma into the epithelium can be interpreted as a reaction to hypoxia or deficiency of any other substance transported via the bloodstream. Apparently, the perivascular connective tissue can be used by ingrowing fibroblasts as a migration pathway. The migrating fibroblasts appear to use the defects of the epithelial basal lamina (whether partially or complete) in order to reach the intercellular space. It is possible that collagen fibrils in the epithelial intercellular space have been laid down by fibroblasts which contribute to the pathological dedifferentiation of the conjunctival epithelium. Keywords: pterygium; ultrastructure; epithelium; blood vessels
Full Text
The Full Text of this article is available as a PDF (250.8 KB).
Figure 1 .
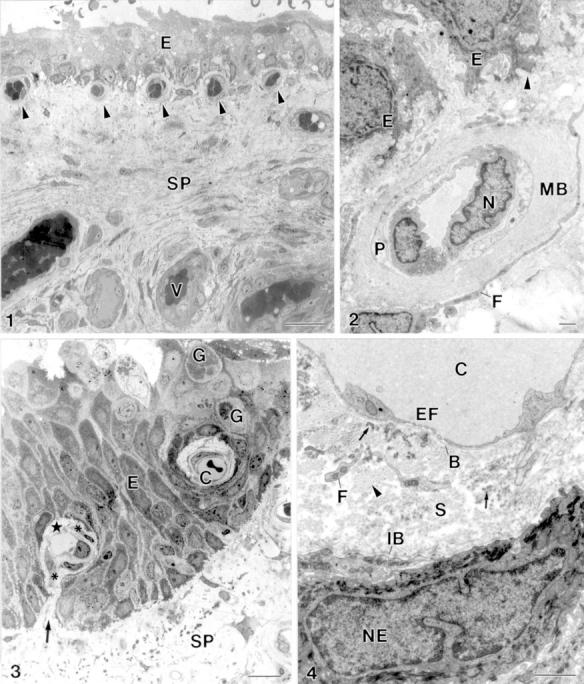
(1) The stroma of the pterygium (SP) is highly vascularised (V). Subepithelial capillary (arrowhead), epithelium (E). Bar = 20 µm. (2) Subepithelial capillary. Subepithelial basal lamina (arrowhead), epithelial cell (E), cellular extension of a fibroblast (F), multilamellar basal lamina of a capillary (MB), endothelial cell nucleus (N), pericyte (P). Bar = 1 µm. (3) Intraepithelial capillary (C) and a capillary with a hilus-like connection (arrow) between the perivascular connective tissue (star) and the subepithelial stroma of pterygium (SP). Fibroblast (asterisk), goblet cell (G), epithelium (E). Bar = 10 µm. (4) The perivascular stroma (S) of intraepithelial capillaries (C) is outlined by an endothelial basal lamina (B) and an "internal" epithelial basal lamina (IB). The perivascular stroma contains cellular processes of fibroblasts (F), collagen fibrils (arrowhead) and electron dense elastin-like material (arrow). Endothelial cell fenestration (EF), epithelial cell nucleus (NE). Bar = 1 µm.
Figure 2 .
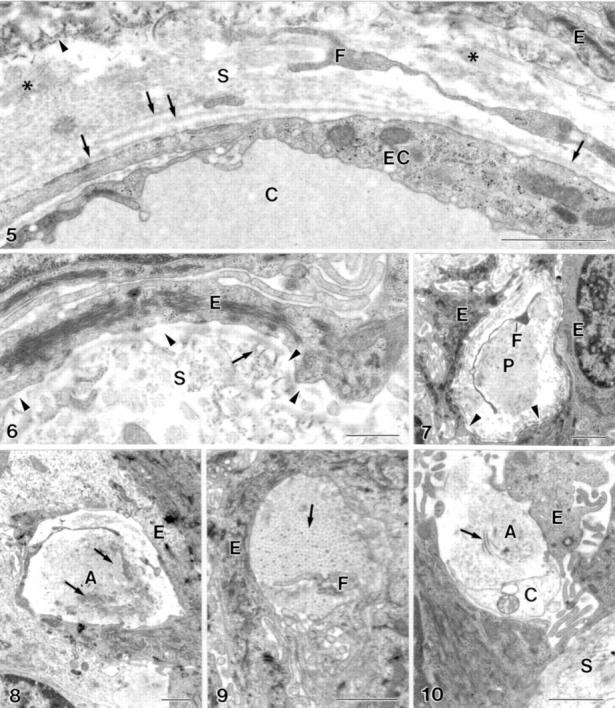
(5) The endothelial basal lamina of the intraepithelial capillaries (C) is partially single layered and partially multilayered (arrow). Anchoring fibrils (arrowhead), wide spaced collagen fibrils (asterisk), epithelial cell (E), endothelial cell (EC), extension of fibroblast (F), perivascular stroma (S). Bar = 1 µm. (6) Defects in the "internal" epithelial basal lamina (arrowhead). Anchoring fibril (arrow), epithelial cell (E), perivascular stroma (S). Bar = 0.5 µm. (7) Defects (arrowhead) in the epithelial basal lamina at a stromal micropapilla (P). Epithelial cell (E), extension of fibroblast (F). Bar = 1 µm. (8-10) The enlarged intercellular space within the epithelium contains structures not typical for epithelia with collagen fibrils (arrow) and fibroblast processes (F). Amorphous material (A), non-identified cell (C), epithelial cell (E), stroma (S). Bar = 1 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brogi E., Wu T., Namiki A., Isner J. M. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994 Aug;90(2):649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- Cameron M. E. Histology of pterygium: an electron microscopic study. Br J Ophthalmol. 1983 Sep;67(9):604–608. doi: 10.1136/bjo.67.9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo M. T. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993 Nov;77(11):734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin P. J., Johnson G. J. Solar ultraviolet radiation and ocular disease: a review of the epidemiological and experimental evidence. Ophthalmic Epidemiol. 1994 Dec;1(3):155–164. doi: 10.3109/09286589409047224. [DOI] [PubMed] [Google Scholar]
- Dushku N., Reid T. W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994 Jul;13(7):473–481. doi: 10.3109/02713689408999878. [DOI] [PubMed] [Google Scholar]
- Hill J. C., Maske R. Pathogenesis of pterygium. Eye (Lond) 1989;3(Pt 2):218–226. doi: 10.1038/eye.1989.31. [DOI] [PubMed] [Google Scholar]
- Hinojosa R., Rodriguez-Echandia E. L. The fine structure of the stria vascularis of the cat inner ear. Am J Anat. 1966 Mar;118(2):631–663. doi: 10.1002/aja.1001180218. [DOI] [PubMed] [Google Scholar]
- Ioachim-Velogianni E., Tsironi E., Agnantis N., Datseris G., Psilas K. HLA-DR antigen expression in pterygium epithelial cells and lymphocyte subpopulations: an immunohistochemistry study. Ger J Ophthalmol. 1995 Mar;4(2):123–129. [PubMed] [Google Scholar]
- Karukonda S. R., Thompson H. W., Beuerman R. W., Lam D. S., Wilson R., Chew S. J., Steinemann T. L. Cell cycle kinetics in pterygium at three latitudes. Br J Ophthalmol. 1995 Apr;79(4):313–317. doi: 10.1136/bjo.79.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok L. S., Coroneo M. T. A model for pterygium formation. Cornea. 1994 May;13(3):219–224. doi: 10.1097/00003226-199405000-00005. [DOI] [PubMed] [Google Scholar]
- Ladoux A., Frelin C. Hypoxia is a strong inducer of vascular endothelial growth factor mRNA expression in the heart. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1005–1010. doi: 10.1006/bbrc.1993.2144. [DOI] [PubMed] [Google Scholar]
- Liu L., Yang D. Immunological studies on the pathogenesis of pterygium. Chin Med Sci J. 1993 Jun;8(2):84–88. [PubMed] [Google Scholar]
- Minchenko A., Bauer T., Salceda S., Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994 Sep;71(3):374–379. [PubMed] [Google Scholar]
- Moran D. J., Hollows F. C. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984 May;68(5):343–346. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohen J. W. Zur funktionellen Morphologie der Conjunctiva. Fortschr Ophthalmol. 1986;83(1):13–24. [PubMed] [Google Scholar]
- Taylor H. R., West S. K., Rosenthal F. S., Munoz B., Newland H. S., Emmett E. A. Corneal changes associated with chronic UV irradiation. Arch Ophthalmol. 1989 Oct;107(10):1481–1484. doi: 10.1001/archopht.1989.01070020555039. [DOI] [PubMed] [Google Scholar]


