Abstract
AIMS—To document the phenotype of an autosomal dominant macular dystrophy diagnosed as having North Carolina macular dystrophy (NCMD) in this British family, and to verify that the disease locus corresponds with that of MCDR1 on chromosome 6q. METHODS—37 family members were examined and the phenotype characterised. DNA samples from the affected members, 19 unaffected and five spouses, were used to perform linkage analysis with six microsatellite marker loci situated within the MCDR1 region of chromosome 6q. RESULTS—Every affected family member had lesions characteristic of NCMD, which developed early in life and usually remain stable thereafter. Although fundus changes are evident in the periphery, all tests revealed that functional loss is restricted to the macula. Some patients with large macular lesions had good visual acuity with fixation at the edge of the lesion at 5° eccentricity. Significant linkage to the MCDR1 locus on chromosome 6q was obtained with three marker loci, with a maximum lod score of 5.9 (q = 0.00) obtained with D6S249. CONCLUSION—This family has the typical phenotype NCMD, and the causative gene was linked to the disease locus (MCDR1) on chromosome 6q. Early onset and localisation of the disease to the central macula allow specialisation of eccentric retina in some eyes with resultant good visual acuity. Keywords: macular dystrophy; linkage analysis; psychophysics
Full Text
The Full Text of this article is available as a PDF (288.4 KB).
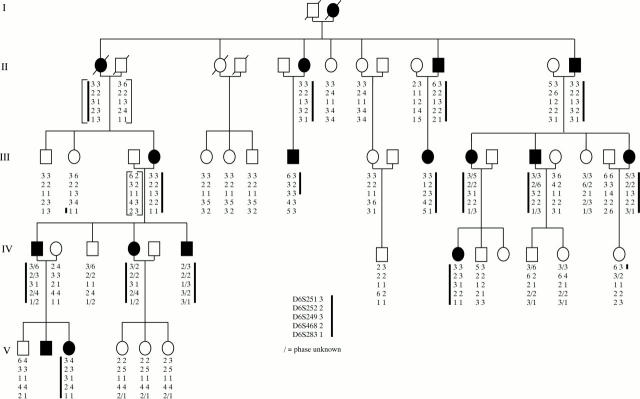
Figure 1 .
Pedigree and haplotype results of six microsatellite marker loci situated on chromosome 6q. Solid symbols indicate affected and open symbols indicate unaffected members of the family. Slashed symbols show deceased individuals. The brackets indicate inferred haplotypes for individuals II-1, II-2, II-13, and III-3. The heavy line indicates the haplotype that appears segregate with disease (a small slash indicates where the phase of the alleles is uncertain). Recombinant IV-13 localises the disease gene telomeric to D6S251 and recombinant III-8 localises the disease gene centromeric to D6S468.
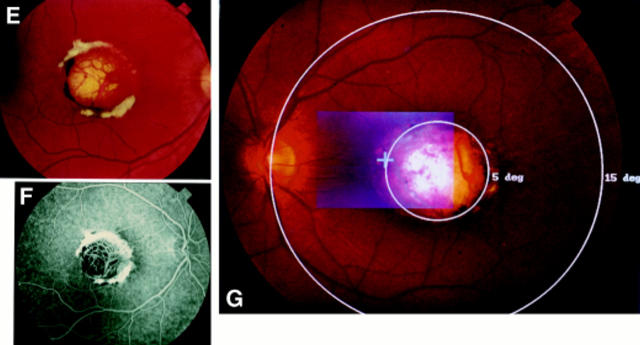
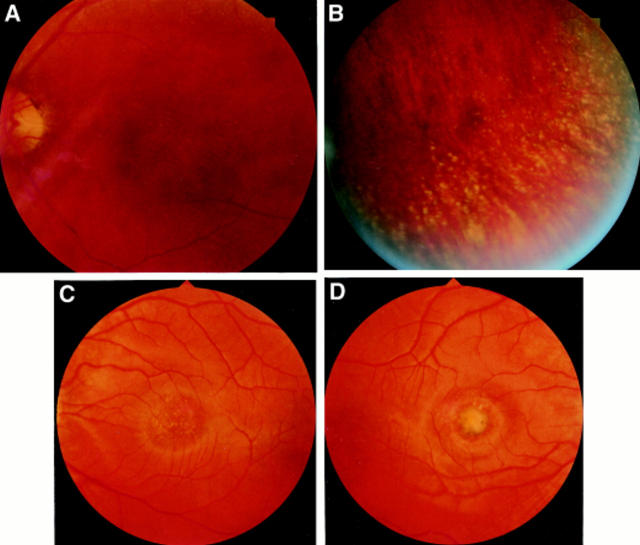
Figure 2 .
A grade I lesion with drusen-like changes in the macular of patient III-8 (A). In the periphery are radial alignment of white dots (B). Patient IV-8 had a grade I lesion in the left eye (C), and a grade II lesion in the right (D). Grade III lesion in patient V-2 showing well demarcated chorioretinal atrophy at the macula, with white fibrous tissue and pigmentation on the edge of the lesion (E, F). An overlay of the SLO guided fixation test and the fundus photography reveals left eye fixation at the nasal rim of the macular lesion at about 5° of eccentricity (G).
Figure 3 .
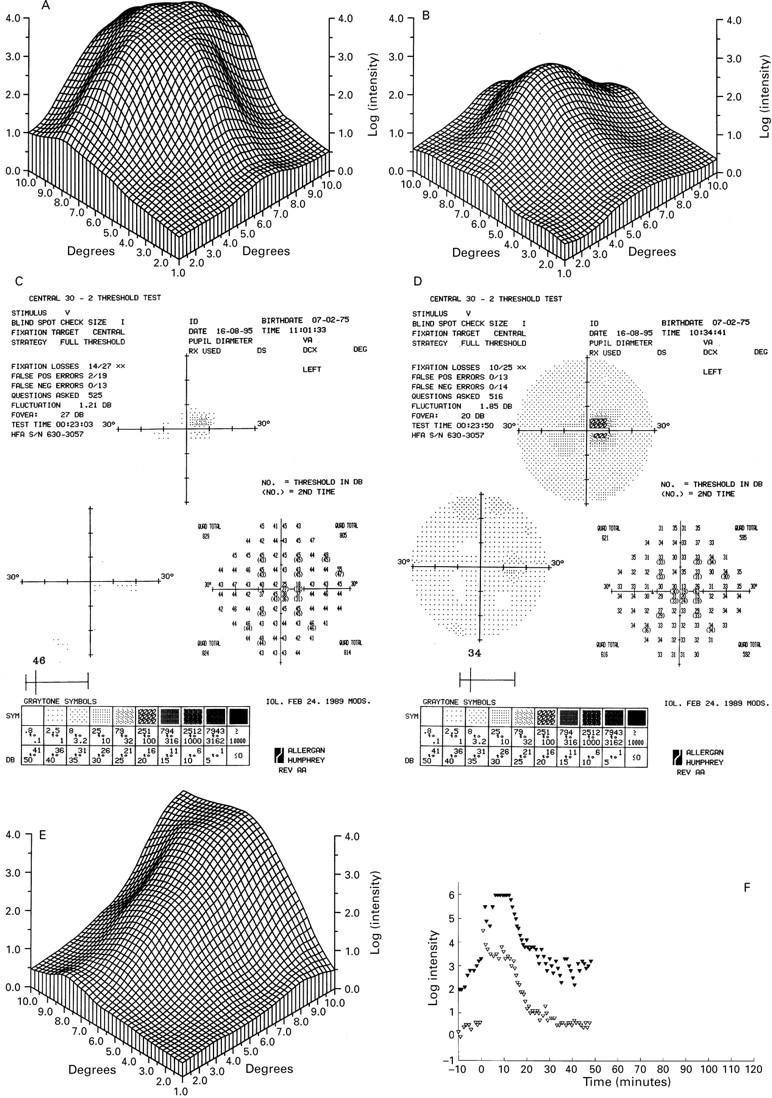
Fine matrix mapping on the left eye of patient IV-8 with a grade II lesion shows up to 3 log units sensitivity loss (A), the right eye with a grade I has normal sensitivities (B). In patient V-2 with a grade III lesion, perimetric tests show a sharply demarcated central sensitivity loss of up to 20 dB in the scotopic (C) and photopic function (D) and displacement of the blind spot. There was marked sensitivity loss on fine matrix mapping (E). Dark adaptation curve reveals elevated prebleach sensitivities at the edge of the scotoma (closed symbols), normal sensitivities at 9° (open symbols), and normal recovery kinetics (F).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Barrada A., Kelsey J. H. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962 Aug;46(8):449–467. doi: 10.1136/bjo.46.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden G. B., Carter R. M., Hogg C. R., Powell D. J., Ernst W. J., Clover G. M., Lyness A. L., Quinlan M. P. A modified ERG technique and the results obtained in X-linked retinitis pigmentosa. Br J Ophthalmol. 1983 Jul;67(7):419–430. doi: 10.1136/bjo.67.7.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood J., Bryant S. A computer program to make linkage analysis with LIPED and LINKAGE easier to perform and less prone to input errors. Ann Hum Genet. 1988 Jul;52(Pt 3):259–259. doi: 10.1111/j.1469-1809.1988.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Fitzke F. W., Pauleikhoff D., Bird A. C. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992 Feb;33(2):334–340. [PubMed] [Google Scholar]
- Frank H. R., Landers M. B., 3rd, Williams R. J., Sidbury J. B. A new dominant progressive foveal dystrophy. Am J Ophthalmol. 1974 Dec;78(6):903–916. doi: 10.1016/0002-9394(74)90800-9. [DOI] [PubMed] [Google Scholar]
- Gregory C. Y., Evans K., Wijesuriya S. D., Kermani S., Jay M. R., Plant C., Cox N., Bird A. C., Bhattacharya S. S. The gene responsible for autosomal dominant Doyne's honeycomb retinal dystrophy (DHRD) maps to chromosome 2p16. Hum Mol Genet. 1996 Jul;5(7):1055–1059. doi: 10.1093/hmg/5.7.1055. [DOI] [PubMed] [Google Scholar]
- Héon E., Piguet B., Munier F., Sneed S. R., Morgan C. M., Forni S., Pescia G., Schorderet D., Taylor C. M., Streb L. M. Linkage of autosomal dominant radial drusen (malattia leventinese) to chromosome 2p16-21. Arch Ophthalmol. 1996 Feb;114(2):193–198. doi: 10.1001/archopht.1996.01100130187014. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Voigt W. J., Parel J. M., Apáthy P. P., Nghiem-Phu L., Myers S. W., Patella V. M. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986 Dec;93(12):1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- Joshi A. R., Mullen L., Small K. W. The retina: genetic studies of several retinopathies located on the short arm of chromosome 17. Curr Opin Neurol. 1997 Feb;10(1):31–35. [PubMed] [Google Scholar]
- Kaplan J., Gerber S., Larget-Piet D., Rozet J. M., Dollfus H., Dufier J. L., Odent S., Postel-Vinay A., Janin N., Briard M. L. A gene for Stargardt's disease (fundus flavimaculatus) maps to the short arm of chromosome 1. Nat Genet. 1993 Nov;5(3):308–311. doi: 10.1038/ng1193-308. [DOI] [PubMed] [Google Scholar]
- Kelsell R. E., Godley B. F., Evans K., Tiffin P. A., Gregory C. Y., Plant C., Moore A. T., Bird A. C., Hunt D. M. Localization of the gene for progressive bifocal chorioretinal atrophy (PBCRA) to chromosome 6q. Hum Mol Genet. 1995 Sep;4(9):1653–1656. doi: 10.1093/hmg/4.9.1653. [DOI] [PubMed] [Google Scholar]
- Kim R. Y., Dollfus H., Keen T. J., Fitzke F. W., Arden G. B., Bhattacharya S. S., Bird A. C. Autosomal dominant pattern dystrophy of the retina associated with a 4-base pair insertion at codon 140 in the peripherin/RDS gene. Arch Ophthalmol. 1995 Apr;113(4):451–455. doi: 10.1001/archopht.1995.01100040067029. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefler W. H., Wadsworth J. A., Sidbury J. B., Jr Hereditary macular degeneration and amino-aciduria. Am J Ophthalmol. 1971 Jan;71(1 Pt 2):224–230. doi: 10.1016/0002-9394(71)90394-1. [DOI] [PubMed] [Google Scholar]
- Millodot M. Foveal and extra-foveal acuity with and without stabilized retinal images. Br J Physiol Opt. 1966;23(2):75–106. [PubMed] [Google Scholar]
- Paschen W., Blackstone C. D., Huganir R. L., Ross C. A. Human GluR6 kainate receptor (GRIK2): molecular cloning, expression, polymorphism, and chromosomal assignment. Genomics. 1994 Apr;20(3):435–440. doi: 10.1006/geno.1994.1198. [DOI] [PubMed] [Google Scholar]
- Peng Y. W., Blackstone C. D., Huganir R. L., Yau K. W. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience. 1995 May;66(2):483–497. doi: 10.1016/0306-4522(94)00569-q. [DOI] [PubMed] [Google Scholar]
- Sander T., Janz D., Ramel C., Ross C. A., Paschen W., Hildmann T., Wienker T. F., Bianchi A., Bauer G., Sailer U. Refinement of map position of the human GluR6 kainate receptor gene (GRIK2) and lack of association and linkage with idiopathic generalized epilepsies. Neurology. 1995 Sep;45(9):1713–1720. doi: 10.1212/wnl.45.9.1713. [DOI] [PubMed] [Google Scholar]
- Small K. W., Hermsen V., Gurney N., Fetkenhour C. L., Folk J. C. North Carolina macular dystrophy and central areolar pigment epithelial dystrophy. One family, one disease. Arch Ophthalmol. 1992 Apr;110(4):515–518. doi: 10.1001/archopht.1992.01080160093040. [DOI] [PubMed] [Google Scholar]
- Small K. W., Killian J., McLean W. C. North Carolina's dominant progressive foveal dystrophy: how progressive is it? Br J Ophthalmol. 1991 Jul;75(7):401–406. doi: 10.1136/bjo.75.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small K. W. North Carolina macular dystrophy, revisited. Ophthalmology. 1989 Dec;96(12):1747–1754. doi: 10.1016/s0161-6420(89)32655-8. [DOI] [PubMed] [Google Scholar]
- Small K. W., Puech B., Mullen L., Yelchits S. North Carolina macular dystrophy phenotype in France maps to the MCDR1 locus. Mol Vis. 1997 Jan 2;3:1–1. [PubMed] [Google Scholar]
- Small K. W., Weber J. L., Roses A., Lennon F., Vance J. M., Pericak-Vance M. A. North Carolina macular dystrophy is assigned to chromosome 6. Genomics. 1992 Jul;13(3):681–685. doi: 10.1016/0888-7543(92)90141-e. [DOI] [PubMed] [Google Scholar]
- Small K. W., Weber J., Roses A., Pericak-Vance P. North Carolina macular dystrophy (MCDR1). A review and refined mapping to 6q14-q16.2. Ophthalmic Paediatr Genet. 1993 Dec;14(4):143–150. doi: 10.3109/13816819309042913. [DOI] [PubMed] [Google Scholar]
- Steinmetz R. L., Garner A., Maguire J. I., Bird A. C. Histopathology of incipient fundus flavimaculatus. Ophthalmology. 1991 Jun;98(6):953–956. doi: 10.1016/s0161-6420(91)32197-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz R. L., Haimovici R., Jubb C., Fitzke F. W., Bird A. C. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993 Sep;77(9):549–554. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R. L., Polkinghorne P. C., Fitzke F. W., Kemp C. M., Bird A. C. Abnormal dark adaptation and rhodopsin kinetics in Sorsby's fundus dystrophy. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1633–1636. [PubMed] [Google Scholar]
- Stone E. M., Nichols B. E., Kimura A. E., Weingeist T. A., Drack A., Sheffield V. C. Clinical features of a Stargardt-like dominant progressive macular dystrophy with genetic linkage to chromosome 6q. Arch Ophthalmol. 1994 Jun;112(6):765–772. doi: 10.1001/archopht.1994.01090180063036. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994 Dec;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Weingeist T. A., Kobrin J. L., Watzke R. C. Histopathology of Best's macular dystrophy. Arch Ophthalmol. 1982 Jul;100(7):1108–1114. doi: 10.1001/archopht.1982.01030040086016. [DOI] [PubMed] [Google Scholar]
- Weinstein G. W., Arden G. B., Hitchings R. A., Ryan S., Calthorpe C. M., Odom J. V. The pattern electroretinogram (PERG) in ocular hypertension and glaucoma. Arch Ophthalmol. 1988 Jul;106(7):923–928. doi: 10.1001/archopht.1988.01060140069027. [DOI] [PubMed] [Google Scholar]
- Wells J., Wroblewski J., Keen J., Inglehearn C., Jubb C., Eckstein A., Jay M., Arden G., Bhattacharya S., Fitzke F. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993 Mar;3(3):213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]
- Wijesuriya S. D., Evans K., Jay M. R., Davison C., Weber B. H., Bird A. C., Bhattacharya S. S., Gregory C. Y. Sorsby's fundus dystrophy in the British Isles: demonstration of a striking founder effect by microsatellite-generated haplotypes. Genome Res. 1996 Feb;6(2):92–101. doi: 10.1101/gr.6.2.92. [DOI] [PubMed] [Google Scholar]
- Zhang K., Bither P. P., Park R., Donoso L. A., Seidman J. G., Seidman C. E. A dominant Stargardt's macular dystrophy locus maps to chromosome 13q34. Arch Ophthalmol. 1994 Jun;112(6):759–764. doi: 10.1001/archopht.1994.01090180057035. [DOI] [PubMed] [Google Scholar]
- von Rückmann A., Fitzke F. W., Bird A. C. In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol. 1997 May;115(5):609–615. doi: 10.1001/archopht.1997.01100150611006. [DOI] [PubMed] [Google Scholar]