Abstract
This study addresses the issue of the effect of immunomodulating therapies in the target organ—the central nervous system (CNS)—in the case of multiple sclerosis. Copolymer 1 (Cop 1, Copaxone, glatiramer acetate), an approved drug for the treatment of multiple sclerosis, is a potent inducer of Th2 regulatory cells in both mice and humans. Highly reactive Cop 1-specific T cell lines that secrete IL-4, IL-5, IL-6, IL-10, and transforming growth factor-β in response to Cop 1 and crossreact with myelin basic protein (MBP) at the level of Th2 cytokine secretion were established from both brains and spinal cords of Cop 1-treated mice. In contrast, no reactivity to the control antigen lysozyme could be obtained in lymphocytes isolated from CNS of mice injected with lysozyme. Adoptively transferred labeled Cop 1-specific suppressor cells were found in brain sections 7 and 10 days after their injection to the periphery, whereas lysozyme-specific cells were absent in the CNS. Hence, Cop 1-induced Th2 cells cross the blood–brain barrier and accumulate in the CNS, where they can be stimulated in situ by MBP and thereby exert therapeutic effects in the diseased organ. This therapeutic effect was manifested, in brains of experimental autoimmune encephalomyelitis-induced mice, by a decrease in the inflammatory cytokine interferon-γ and by secretion of the anti-inflammatory cytokine IL-10 in response to the autoantigen MBP.
Current therapies attempt to interfere with pathological processes by immune intervention. However, whereas the peripheral effect of these therapies was extensively studied, little information exists on their action in the target organ. In this respect, immunomodulation of autoimmune diseases of the central nervous system (CNS) is especially interesting, as the accessibility of immune factors and regulatory cells to the CNS through the blood–brain barrier (BBB) has a crucial role in determining its success. Lack of complete lymphatic drainage and incomplete graft rejection supported the concept that the CNS is inaccessible to circulating lymphocytes (1). This notion has been re-evaluated with the availability of T cell lines that, upon injection outside the target tissue, can induce inflammatory response inside the CNS. There is currently a consensus that the CNS is routinely patrolled by activated lymphocytes that cross the BBB regardless of their specificity. Yet, T cells specific for non-neural antigens migrate out of the CNS or die by apoptosis, whereas T cells that recognize CNS antigens can be stimulated in situ, thus leading to their proliferation and accumulation in the CNS (1–4). In this study, we used copolymer 1 (Cop 1, Copaxone, glatiramer acetate), an approved immunomodulating drug for the treatment of multiple sclerosis (MS) (5–7), to study how a drug delivered in the periphery can induce down-regulation of autoimmune pathological processes in the CNS and consequently generate therapeutic effect.
MS and its animal model experimental autoimmune encephalomyelitis (EAE) are autoimmune inflammatory and demyelinating diseases of the CNS. Whereas Th1 cells, which produce IL-2, interferon (IFN)-γ, and tumor necrosis factor, have been implicated in the pathological processes, Th2/Th3 cells that secrete IL-4, IL-5, IL-10 and transforming growth factor (TGF)-β are involved in down-regulation of these diseases (8, 9). Local cytokine production varies significantly during the disease progress, and changes in discrete sets of cytokines are associated with acute response or recovery/chronic phases of the disease (8–11). In this regard, the Th1/Th2 balance has a key role in MS/EAE regulation. Yet, T cell migration to the CNS and their operation in situ was predominantly studied by testing the ability of Th1 encephalitogenic T cells to penetrate the BBB and induce disease (1–4), whereas the ability of Th2 cells to enter into the CNS and induce there anti-inflammatory effect is still controversial.
We have previously demonstrated that the unresponsiveness to EAE induced by Cop 1 is mediated by Th2 suppressor cells that secrete high amounts of IL-4, IL-5, IL-6, and IL-10 in response to Cop 1 but do not secrete IL-2, IFN-γ, or tumor necrosis factor-α (5, 12–14). Notably, the Cop 1-induced Ts cells crossreact with myelin basic protein (MBP), which is a principal autoantigen in both EAE and MS, by secretion of Th2 immunosuppressive cytokines. They were also able to induce bystander suppression on processes that are mediated by other myelin antigens, such as proteolipid protein (15). A shift from Th1-biased cytokine profile toward a Th2-biased profile was recently observed in Cop 1-treated MS patients (16–18). It is therefore likely that Cop 1-specific cells of the Th2 type, similar to those demonstrated in the mouse system, are involved in the therapeutic effect induced by Cop 1 in MS. However, Cop 1-induced Th2 regulatory cells have been demonstrated only in the periphery (spleen and lymph nodes of experimental animals or peripheral blood mononuclear cells in humans). There is no information on whether these cells can reach the organ in which the pathological processes of EAE or MS occur—the CNS. It is reasonable to assume that if these cells are relevant to the therapeutic effect induced by Cop 1, they should be present in the diseased organ. The goal of the present study was therefore to find out whether the Cop 1-specific cells induced in the periphery can pass the BBB and persist in the CNS. This was attempted by using two approaches: 1) isolation of Cop 1-specific Th2 cells from the CNS of Cop 1-treated mice, 2) following the appearance in the CNS of adoptively transferred labeled Cop 1-specific cells. We report that indeed Cop 1-specific Th2 cells could be obtained from the CNS of Cop 1-treated mice, and adoptively transferred Cop 1-specific cells could be demonstrated in the brain. Furthermore, treatment with Cop 1 induced a Th1 to Th2 cytokine shift in the brain of EAE-induced mice, thus confirming for the first time the direct linkage between the therapeutic activity of Cop 1 in EAE/MS and its immunomodulatory effect in the diseased organ—the CNS.
Materials and Methods
Mice.
(SJL/JxBALB/c) F1 mice were purchased from Jackson Laboratories. Female mice, 8–16 weeks old, were used in all experiments.
Antigens.
Copolymer 1 consists of acetate salts of synthetic polypeptides, containing four naturally occurring amino acids: L-alanine, L-glutamate, L-lysine, and L-tyrosine (19). Two Cop 1 batches obtained from Teva Pharmaceutical Industries were used throughout the study: batches 242994699 and 242990599, with average molecular masses of 7,050 and 73,00 kDa, respectively. Myelin basic protein was isolated from spinal cords of mice, as previously described (20). Lysozyme from egg white was obtained from Sigma.
Lymphocyte Isolation from the CNS.
Mice were rendered resistant to EAE by prior injection of Cop 1 (10 mg per mouse in incomplete Freund's adjuvant). Control mice were similarly injected with lysozyme. Each group contained 15–20 animals. In two experiments, mice were challenged for EAE, 20 days postimmunization, by spinal cord homogenate (2 mg per mouse in complete Freund's adjuvant) followed by two injections of pertussis toxin 0.2 μg per mouse. In two additional experiments, mice were not induced with EAE. One month after the injection of Cop 1 or lysozyme, the mice were anesthetized and perfused intracardially with 50 ml of cold PBS. Brains, spinal cords, and spleens were excised and dissociated to single-cell suspensions. CNS lymphocyte populations were isolated by separation on Percoll gradient (Amersham Pharmacia), collecting the fraction between 30 and 60%. This fraction is referred to as whole lymphocyte population.
Reactivity Assays.
Freshly isolated CNS cells (1 × 105), spleen cells (5 × 105), or T cell lines (1 × 104) were cultured with irradiated (3,000 rad) spleen cells (5 × 105), as well as with the indicated antigens, in a final volume of 250 μl per well in triplicates. For proliferation, assay cultures were pulsed with 1 μCi of [3H]thymidine after 48 h of incubation and harvested 6–12 h later. For cytokine assays, culture supernatants (50 μl from each well) were collected at 24 h (for IL-2, IL-6, and IFN-γ), 48 h (for IL-4, IL-5, and IL-10), or 72 h (for TGF-β) and tested by ELISA using mAb pairs from PharMingen. Threshold detection was less than 1 pg/ml.
T Cell Lines.
The isolated cells were cultured and selected in vitro using Cop 1 (50 μg/ml) or lysozyme (100 μg/ml) with irradiated spleen cells (5 × 106/ml) in culture medium (RPMI medium 1640 with 1% autologous serum or 10% FCS). After 4 days, cells were transferred to culture medium supplemented with 10% supernatant of Con A activated spleen cells as T cell growth factor. Every 10–21 days, cells were stimulated by exposure to the antigen on irradiated spleen cells followed by propagation in T cell growth factor medium (14).
Cell Staining.
Three days following antigen stimulation, activated cells were labeled with the fluorescent dye Hoechst (Molecular Probes), which binds to the cell nucleus. A sample of labeled cells was maintained in culture and monitored after 3 and 6 weeks by fluorescence microscopy to ascertain the presence of the dye, and by proliferation and cytokine secretion to ensure their functional activity.
Adoptive Transfer.
Activated labeled cells of Cop 1 or lysozyme-specific lines (30 × 106 per mouse) were injected intraperitoneally to normal mice with or without injection of pertussis toxin. Brains were removed at different time points, fixed in paraformaldehyde, and sectioned by cryostat. Perfusion was performed when indicated. From each brain at least 50 sections (40 μm) were examined for the presence of fluorescent cells.
Results
Reactivity and Cytokine Secretion by Cells Isolated from Brains of Mice Pretreated with Cop 1 or Lysozyme.
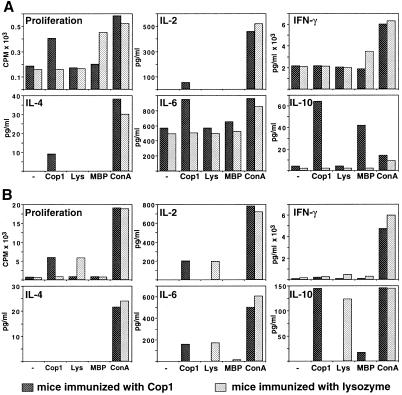
To investigate whether Cop 1-specific cells induced in the periphery can be demonstrated in the CNS, lymphocytes were isolated from brains and spinal cords of mice that had been rendered resistant to EAE by prior treatment with Cop 1. Control animals were similarly injected with lysozyme. To find out whether migration of lymphocytes into the CNS is dependent on EAE induction, parallel experiments were conducted in naive mice and in those challenged for disease by subsequent injection of spinal cord homogenate. One month after the injection of Cop 1 or lysozyme, the mice were perfused intracardially, brains and spinal cords were excised, and the lymphocyte populations were isolated and evaluated for their reactivity and specificity by proliferation and cytokine response. In all of the experiments, the yield of CNS lymphocytes was very low, although higher numbers of lymphocytes (1 × 105 cells per brain) were obtained from EAE-induced mice than from mice that were not challenged for EAE (0.5 × 105 cells per brain). However, even though 5-fold less brain cells than spleen cells were available for culture in each tested sample, proliferation and cytokine secretion could still be detected in cultures of brain lymphocytes (Fig. 1, Table 1).
Figure 1.
Proliferation and cytokine secretion by cells isolated from EAE-induced mice pretreated with Cop 1 (dark bars) or lysozyme (light bars). Reactivity of whole lymphocyte populations isolated from brains (A) and spleens (B) is demonstrated. Mice rendered resistant to EAE by injection of Cop 1 (10 mg per mouse in incomplete Freund's adjuvant) and control mice similarly injected with lysozyme were challenged for disease with spinal cord homogenate. One month later, the mice were perfused, and whole lymphocyte population isolated from brains and spleens was evaluated for their reactivity. Proliferation was measured by thymidine incorporation (cpm) into triplicate cultures and cytokine secretion (pg/ml) by quantitative ELISA in duplicates. The response to medium alone (−), Cop 1 (50 μg/ml), MBP (100 μg/ml), and Con A (5 μg/ml) was tested. Standard deviations were under 20% of the mean. Results represent one of four independent experiments.
Table 1.
Proliferation and cytokine secretion by whole lymphocyte populations, from brains of Cop 1 immunized mice, with and without EAE induction
| EAE challenge | Antigen | Proliferation, cpm | Cytokines, pg/ml
|
|||||
|---|---|---|---|---|---|---|---|---|
| IL-2 | IFN-γ | IL-4 | IL-5 | IL-6 | IL-10 | |||
| + | − | 183 | − | 2,169 | − | − | 568 | 4 |
| + | Cop 1 | 406 | 53 | 2,166 | 9 | − | 944 | 64 |
| + | MBP | 198 | − | 1,850 | − | − | 653 | 42 |
| + | Con A | 582 | 458 | 6,012 | 38 | − | 961 | 14 |
| − | − | 104 | − | 136 | − | − | 73 | − |
| − | Cop 1 | 301 | − | 143 | 29 | 123 | 154 | 165 |
| − | MBP | 126 | − | 134 | − | − | 86 | 77 |
| − | Con A | 499 | 235 | 264 | 31 | 123 | 104 | 41 |
(SJL × BALB/c)F1 mice that had been rendered resistant to EAE by injection of Cop 1 (10 mg per mouse in ICFA) were challenged or unchallenged for disease, 3 weeks later, with spinal cord homogenate. One month after immunization with Cop 1, mice were perfused intracardially and brains were harvested. The isolated lymphocytes were evaluated for their reactivity by measuring proliferation response by thymidine incorporation in triplicate cultures, and cytokine secretion by quantitative ELISA in duplicates. Reactivity to medium alone, Cop 1 50 μg/ml, MBP 100 μg/ml, and Con A 1 μg/ml, was tested. Standard deviations were under 20% of the mean. Results represent one of four independent isolation experiments.
A specific reactivity to Cop 1 was manifested by cells isolated from brains of mice pretreated with Cop 1 (Fig. 1A, Table 1). This was manifested by proliferation and by cytokine secretion i.e., IL-2, IL-4, IL-6, and IL-10 in brain cells isolated from EAE-induced mice and IL-4, IL-5, IL-6, and IL-10 in brain cells obtained from mice that were not induced with disease. In the case of IL-10 secretion, significant crossreactivity with MBP was also apparent. There was no specific secretion of IFN-γ in response to Cop 1 over the background secretion with medium alone. In contrast to the responses to Cop 1 in brains of mice pretreated with Cop 1, no specific reactivity to lysozyme could be observed in the brains of the control group, either by proliferation or by cytokine secretion (Fig. 1A). Notably, spleen cells of lysozyme-injected mice responded to lysozyme, by both proliferation and cytokine secretion (Fig. 1B). The level of the responses in the spleens to Cop 1 or lysozyme was at the same order of magnitude, indicating that although both Cop 1 and lysozyme induced specific reactivity in the periphery, only Cop 1 induced specific response in the CNS.
Cells from brains of the EAE-induced mice that had been preexposed to lysozyme responded to the autoantigen MBP by secretion of a high amount of INF-γ (3491 pg/ml) and by proliferation (2.9 stimulation index). In contrast, the amount of INF-γ secreted in response to MBP by cells from brains of Cop 1-pretreated and EAE-induced mice was significantly lower (1,850 pg/ml), similar to the background, and no proliferation response to MBP was manifested by these cells (Fig. 1A). On the other hand, the cells that originated in brains of Cop 1-treated mice secreted significant amounts of IL-10 upon stimulation with MBP, whereas no IL-10 secretion in response to MBP could be detected in cells from brains of lysozyme-injected mice. Interestingly, the CNS lymphocyte populations from either the Cop 1 or the lysozyme-treated mice, which were isolated following the subsequent induction of EAE, secreted excessive amounts of IFN-γ and IL-6 even without any stimulation when incubated with medium alone (Fig. 1A), whereas the secretion of these cytokines by spleen cells was considerably lower (Fig. 1B). This intense secretion of IFN-γ and IL-6 was not found in CNS cells from mice in which EAE was not induced (Table 1).
Reactivity and Cytokine Secretion by in Vitro Stimulated Cells Isolated from Brains (Short-Term Lines).
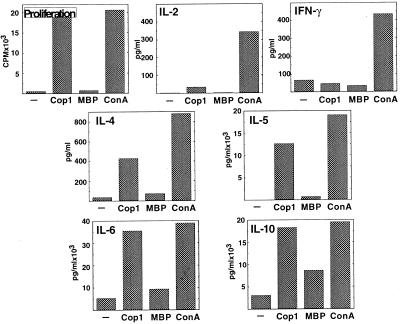
The CNS isolated cells were cultured in vitro with the immunizing antigens to establish T cell lines. Although no cell growth was obtained in cultures originated in CNS of lysozyme-pretreated mice after stimulation with either the immunizing antigen lysozyme or with Cop 1, a 2-fold growth was obtained already after one stimulation cycle with Cop 1 in cultures originated in CNS of Cop 1-treated mice. The reactivity of a representative short-term line, Cop-Br-1, generated from brains of Cop 1-treated and EAE-induced mice after one stimulation with Cop 1 is demonstrated in Fig. 2. A high specific proliferation response to Cop 1 was exhibited already at this stage (43 stimulation index). Only low amounts of IL-2 and no IFN-γ were secreted in response to Cop 1. Thus, the high levels of IFN-γ that had been secreted by whole lymphocyte population from brains of EAE-induced mice (the background secretion as well as the response to the mitogen Con A, Fig. 1A) were mostly diminished after one stimulation cycle with Cop 1 (Fig. 2). In contrast to the Th1 cytokines, high amounts of Th2 cytokines IL-4, IL-5, IL-6, and IL-10 were secreted in response to the stimulating antigen Cop 1 as well as to Con A. These amounts were much higher than the amounts of Th2 cytokines secreted by whole CNS population (Fig. 1A and Table 1). The IL-4 and IL-10 secretions in response to Cop 1 were elevated more than 30-fold, and IL-5, which was not detected at all at the level of whole CNS population, was secreted at a significant level (1265 pg/ml) by the short-term line. Hence, a highly reactive Cop 1-specific Th2 line was obtained already after one cycle of in vitro exposure to Cop 1. Although Th1 cytokine secretion or proliferation could not be detected when the Cop 1-specific short-term line was incubated with MBP, crossreactivity was observed by Th2 cytokine secretion, i.e., IL-4 (98 pg/ml, 23% crossreactivity), IL-6 (933 pg/ml, 26% crossreactivity), and IL-10 (860 pg/ml, 47% crossreactivity). Thus, the crossreactivity with MBP, which had been demonstrated in the freshly isolated lymphocytes only by IL-10 secretion, was demonstrated now at the level of additional Th2 cytokines.
Figure 2.
Proliferation and cytokine secretion by the short-term line Cop-Br-1 originated from brains of EAE-induced mice pretreated with Cop 1, after one in vitro stimulation with Cop 1. Proliferation (cpm) into triplicate cultures and cytokine secretion (pg/ml) in duplicates were measured in response to medium alone (−), Cop 1 (50 μg/ml), MBP (100 μg/ml), and Con A (5 μg/ml). Standard deviations were under 20% of the mean.
Reactivity and Cytokine Secretion by T Cell Lines Isolated from Brains and Spinal Cords (Long-Term Lines).
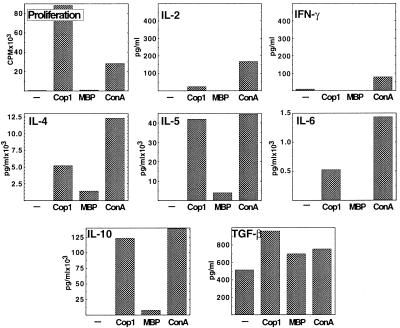
Established (long-term) lines could also be generated from brains of Cop 1-treated mice. These lines exhibited even higher proliferation response to Cop 1 than the short-term lines (150 stimulation index by the representative line Cop-Br-1 after three stimulations with Cop 1, Fig. 3). The levels of IL-2 and IFN-γ (the background as well as the secretion in response to Cop 1 or Con A) continued to decline, and the level of IL-6 was also reduced in comparison to the short-term line (Fig. 2). On the other hand, marked elevation in the other Th2 cytokines IL-4, IL-5, and IL-10 was exhibited (in the case of IL-10, more than 12 ng/ml were secreted in response to Cop 1, Fig. 3). TGF-β was also secreted by the long-term line to a significant level. The Th2 cytokines IL-4, IL-5, IL-10, and TGF-β were secreted also in response to MBP (Fig. 3).
Figure 3.
Proliferation and cytokine secretion by the long-term T cell line Cop-Br-1, originated from brains of EAE-induced mice pretreated with Cop 1, after three in vitro stimulations with Cop 1. Proliferation (cpm) into triplicate cultures and cytokine secretion (pg/ml) in duplicates were measured in response to medium alone (−), Cop 1 (50 μg/ml), MBP (100 μg/ml), and Con A (5 μg/ml). Standard deviations were under 20% of the mean.
Cop 1 specific T cell lines could also be generated from spinal cords of the Cop 1-treated mice (data not shown). Similarly to the lines originated from brains, the spinal cord-originated lines exhibited high specific proliferative response and a confined Th2 secretion of IL-4, IL-5, IL-6, IL-10, and TGF-β in response to Cop 1, as well as pronounced crossreactivity with MBP at the level of Th2/Th3 cytokines.
Five different highly reactive T cell lines that secrete high amounts of Th2 cytokines, IL-4, IL-5, IL-6, IL-10, and TGF-β in response to Cop 1 and MBP, were generated from brains or spinal cords of mice treated with Cop 1, with or without EAE preinduction. In contrast, no Cop 1- or lysozyme-specific lines could be obtained from either brain or spinal cord of mice treated with lysozyme either with or without EAE induction even after five in vitro stimulations.
The Presence of Fluorescent-Labeled Adoptively Transferred T Cells in the Brain.
To prove that Cop 1-specific T cells are indeed able to cross the BBB and accumulate in the CNS tissue, we followed the appearance in the brain of adoptively transferred labeled Cop 1-specific T cells. Activated cells of Cop 1-specific lines that had originated from spleens and were subsequently confirmed as suppressor lines by suppression of EAE in vivo (14, 15) were labeled with the fluorescent dye Hoechst, which binds to the cell nucleus. A sample of labeled cells was maintained in culture and monitored after 3 and 6 weeks by fluorescence microscopy (to ascertain the presence of the dye) and by proliferation and cytokine secretion (to ensure their functional activity). Hoechst labeling did not interfere essentially with the biological activity of the cells as demonstrated by comparing proliferation and cytokine secretion of labeled cells to unlabeled cells (data not shown), as well as by the ability of the labeled cells to prevent EAE induction similarly to unlabeled cells (0% disease incidence in mice injected with labeled or unlabeled cells in comparison with 60% disease incidence in untreated mice).
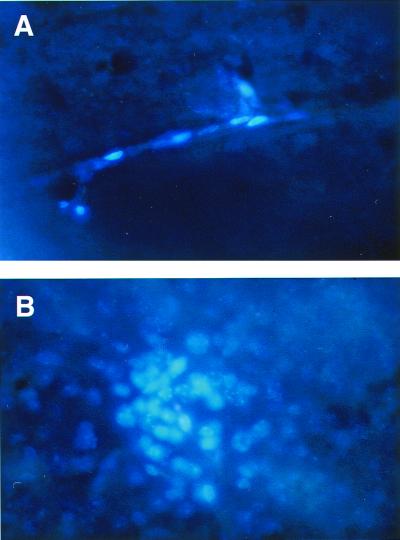
The labeled cells were injected intraperitoneally to normal mice with subsequent injection of pertussis toxin. Brains were removed at different time points after cell injection, sectioned, and examined for the presence of fluorescent cells. As depicted in Fig. 4, the presence of the fluorescent cells is clearly demonstrated in brain sections obtained from mice 7 days after the injection of labeled Cop 1-specific T cell line. In some of the sections, the labeled cells were visible in the blood vessels (Fig. 4A), and in most of the sections, clusters of labeled cells were observed and penetration to the brain tissue was definitely visualized (Fig. 4B). To ensure that the labeled Cop 1-specific cells indeed crossed the BBB and migrated out of the blood vessels, their presence was examined, 7 days after their injection, in CNS of mice that had been perfused before brain removal. In brain sections of perfused mice, labeled cells were not found in the blood vessels, yet conglomerations of labeled cells were still present in more than 50% of the sections, thus confirming their entry into the CNS tissue.
Figure 4.
Brain sections of mouse adoptively transferred with Cop 1-specific T cells. Activated T cells of the Cop 1-specific line Ts-D were labeled with the fluorescent dye Hoechst and injected intraperitoneally to normal mice with subsequent injection of pertussis toxin. After 7 days, brains were removed, fixed in paraformaldehyde, and sectioned (40 μm). Cells in blood vessels (A) and a cluster of cells in the brain tissue (B) are demonstrated. (×400.)
The presence in the brain of adoptively transferred labeled cells was demonstrated using three different Cop 1 suppressor T cell lines, in comparison to their essential absence in the case of similarly labeled two lysozyme-specific T cell lines (Table 2). The Cop 1-specific cells were found in most (78–88%) of the brain sections after 3 and 7 days and, in a substantial number (12–58%) of slides, even 10 days after their injection. In contrast, only very few lysozyme-specific T cells could be detected (2 of 150 sections) on day three, and they were completely absent in the CNS after 7 or 10 days.
Table 2.
The presence of fluorescently labeled T cells in the brain following adoptive transfer
| Specificity of line | Line | Percent sections with labeled cells
|
||
|---|---|---|---|---|
| 3 days | 7 days | 10 days | ||
| L-1-4 | 821 | 782 | 121 | |
| Cop 1 | Ts-10 | 882 | 843 | 522 |
| Ts-D | ND | 924 | 582 | |
| Ts-D* | ND | 482 | ND | |
| Ts-D† | ND | 462 | ND | |
| Lysozyme | Lys 1 | 12 | —2 | ND |
| Lys 10 | 21 | —2 | ND | |
Activated T cells of Cop 1 or lysozyme lines were labeled with the fluorescent dye Hoechst. The labeled cells were injected intraperitoneally to normal mice with subsequent injection of pertussis toxin, unless otherwise indicated. Brains were removed at the indicated times, fixed in paraformaldehyde, and sectioned (40 μm). Perfusion was performed when indicated by intracardial injection of 50 ml of PBS. From each brain at least 50 sections were examined for the presence of fluorescently labeled cells. Superscript numbers indicate the number of mice tested. ND, not done.
Mice perfused before brain harvesting.
Without pertussis toxin.
To find out if the Cop 1-activated cells can penetrate through the intact BBB or whether additional facilitating factors are required, their presence in the brain was monitored 7 days after injection and without subsequent pertussis toxin administration. Labeled cells that had been injected to the periphery without pertussis toxin were still found in a considerable number of brain sections (46%) yet in smaller number than in brains of mice injected with the addition of pertussis toxin. This confirms the ability of the Cop 1-specific cells to migrate through the intact BBB even without the effect of auxiliary factors.
Discussion
Immunomodulating therapies for MS and its animal model EAE have been extensively studied. However, understanding of the way by which these therapies operate and especially their effect in the target organ—the CNS—has lagged behind. The ability of a therapeutic agent to reach by itself or generate regulatory factor(s) that will arrive at the site in which the pathological process occurs is obviously essential to its success. The immunomodulator Cop 1, which is one of the few approved drugs for the treatment of MS, has been previously demonstrated as a potent inducer of Th2 regulatory cells in the periphery of both mice and humans (14–18). Here, we show that such Cop 1-induced T cells can reach the MS diseased organ, the CNS, and consequently generate therapeutic effect in situ. This was demonstrated by both the isolation of such cells from CNS of actively sensitized Cop 1-treated mice and their localization in the brain after being passively transferred to the periphery.
A specific reactivity to Cop 1 was found in whole lymphocyte population obtained from brains of Cop 1-treated mice, manifested by proliferation and by cytokine secretion, albeit the responses obtained were low (Fig. 1A, Table 1). It should be noted, however, that the reactivity found in the whole spleen cell population was also low (Fig. 1B), although the number of cells cultured in each spleen sample was 5-fold higher than in the cultures of the lymphocytes originating in the CNS. This low reactivity of freshly isolated cells could result from the low frequency of the specific cells in whole lymphocyte population. To overcome the limitations of small yield and low frequency of antigen-specific cells found at the level of whole lymphocyte population, the isolated cells from the CNS were cultured in vitro to establish T cell lines. The short-term T cell lines originated from CNS of Cop 1-treated mice demonstrated a potent response to the immunizing antigen Cop 1 already after one enrichment cycle, as manifested by both high specific proliferation and secretion of high amounts of Th2 cytokines (Fig. 2). This was even more pronounced after three in vitro stimulations with Cop 1, when highly reactive Cop 1-specific Th2 lines were obtained (Fig. 3). Five different Cop 1-specific T cell lines, which secrete high amounts of the Th2/Th3 cytokines IL-4, IL-5, IL-6, IL-10, and TGF-β, were generated from brains and spinal cords of mice treated with Cop 1 with or without induction of EAE. The ability to establish Cop 1-specific lines was not due to a nonspecific in vitro education artifact because such lines could not be obtained from CNS lymphocytes of control mice treated with lysozyme. Hence, the generation of Cop 1-specific CNS T cell lines resulted from the presence of these specific cells in the CNS of Cop 1-treated mice.
The crossreactivity of Cop 1 with the autoantigen MBP, which had been previously demonstrated for peripheral T cells (14, 15), was observed now also in the CNS. This was manifested by IL-10 secretion in whole lymphocyte population (Fig. 1A, Table 1) and by secretion of substantial amounts of additional Th2/Th3 cytokines (IL-4, IL-5, IL-6, and TGF-β) when highly reactive Cop 1-specific T cell lines were generated (Figs. 2 and 3). Notably, this crossreactivity was confined to the level of Th2 cytokine secretion as neither proliferation nor Th1 cytokine secretion was observed in response to MBP.
In contrast to the Cop 1-specific response demonstrated by CNS lymphocytes and by T cell lines from Cop 1-treated mice, no reactivity to the control antigen lysozyme could be demonstrated in the CNS of mice treated with lysozyme either by proliferation or by cytokine secretion. The lack of response to lysozyme was found in freshly CNS-isolated cells (Fig. 1A), as well as by the inability to grow lysozyme-specific T cell lines from these cells. Yet, freshly isolated spleen cells of lysozyme-injected mice did react with lysozyme in the same order of magnitude as the response to Cop 1 in Cop 1-treated mice (Fig. 1B). Thus, both Cop 1 and lysozyme induce specific reactivity in the periphery, but the lysozyme-specific cells stayed in the periphery and did not reach to the CNS, whereas Cop 1-specific cells did reach to the CNS. This phenomenon was also directly demonstrated by following their appearance in the brain after being adoptively transferred to the peritoneum. The presence of labeled cells from three different Cop 1-specific T cell lines in the brain was clearly demonstrated (Fig. 4, Table 2). Penetration to the brain tissue was definitely shown, and clusters of labeled cells were visualized, suggesting that the cells are in an activated state. In contrast, cells from two lysozyme-specific control lines were completely absent in the CNS after 7 or 10 days from their injection. The entry into the CNS tissue of Cop 1-specific cells was further confirmed by their presence in brains of mice that had been perfused before brain removal. Cop 1-specific cells that had been injected to the periphery without subsequent administration of pertussis toxin were also found in a considerable number of brain sections. Thus, Cop 1-specific activated cells are able to accumulate in the intact BBB without additional facilitating factors. The ability of Cop 1-specific lymphocytes to migrate into the intact CNS was evident also from the ability to obtain a Cop 1-specific response as well as Cop 1-specific lines from CNS of mice that were not preinduced with EAE.
The CNS is not exempt from immune surveillance. There is currently a consensus that activated T cells of any specificity are able to cross the BBB and penetrate into the CNS (1–4). The concentration of such T cells in the CNS was shown to peak between 9 and 24 h from injection, but thereafter T cells that were unable to find their antigen in the CNS returned to baseline levels. In contrast, it was demonstrated that T cells specific to CNS antigens such as MBP stayed in the CNS, and their number peaked around day 6 after injection (4). In the present study, the kinetics found for the lysozyme-specific lines is consistent with the results reported for non-CNS specific cells, whereas that of the Cop 1-specific lines is similar to the kinetics described for cells specific to CNS antigens such as MBP. The “late” presence of T cells in the CNS was shown to be strictly dependent upon recognition of CNS antigens in vivo and was attributed to in situ stimulation by their specific antigen presented on astrocytes or microglia cells (1–4). In the case of the Cop 1-specific T cells, their ability to crossreact with the naturally occurring myelin antigen–MBP, which is an abundant component in the CNS, enabled them to be stimulated in situ and induce their suppressive activity in the diseased organ.
T cell extravasation to the CNS was predominantly studied by testing the ability of Th1 encephalitogenic T cells to penetrate the BBB and induce disease (1–4). The ability of Th2 cells to enter into the CNS and induce there anti-inflammatory effect is more controversial. It was argued that adhesion mechanisms distinguish between the two subsets and mediate selective localization to different tissues and organs (21, 22), whereas others found that Th1 and Th2 cells are attracted to the CNS with similar kinetics (23). Our results clearly indicate that Cop 1-specific Th2 cells migrate and accumulate in the CNS with kinetics similar to that reported for Th1 cells (4, 23), yet they do not exclude the possibility that extravasation of Th2 cells in the brain is less efficient than that of Th1 cells. The capability of Th2 cells to secrete Th2 cytokines in situ after their infiltration to the CNS was severely questioned following the finding that MBP-specific Th2 cells had the potential to induce EAE in immunodeficient mice (23) and our work showing disease aggravation by such lines (15). This effect was attributed to residual Th1 cytokine secretion and to the CNS environment that might induce a biased cytokine profile toward the Th1 pathway (24). In contrast, the Cop 1-induced cells that invaded the CNS continued to exhibit Th2 profile in situ, as demonstrated by the cytokine profile of the T cells obtained from the CNS of Cop 1-treated mice. Cop 1-induced cells crossreact with MBP only at the level of Th2 secretion and thus evoked only suppressive effect in vivo and are devoid of any encephalitogenic activity (9). Hence, Cop 1-specific cells either generated by active immunization with Cop 1 or passively transferred to the periphery accumulate in the CNS. Furthermore, they maintain their Th2 phenotype in situ and secrete anti-inflammatory cytokines, which induce a therapeutic effect in the diseased organ.
The in situ therapeutic activity induced by the Cop 1-specific cells was manifested in the modification of response of CNS lymphocytes to the encephalitogen MBP. Although CNS lymphocytes from the control mice exhibited specific proliferation and INF-γ secretion in response to MBP, Cop 1 treatment abrogated the proliferation and induced a cytokine shift in the response to this autoantigen (Fig. 1A). This cytokine shift was exhibited by abolishment of the INF-γ secretion and induction of IL-10 secretion. It is of interest that parallel results were observed in the case of another autoimmune disease, diabetes mellitus. In that case, an in situ therapeutic activity of hsp60 peptide was manifested in the target organ (the islet of diabetic mice) by decrease in IFN-γ, yet it was not accompanied by increase in Th2 cytokines (25). IFN-γ is a principal Th1 effector cytokine involved in the pathological processes of EAE and MS, and its elevation is typical to disease exacerbation (8–11). Interestingly, high background secretion of IFN-γ, even without any stimulation, was found in this study in brain lymphocytes of EAE-induced mice (Fig. 1A). This intense IFN-γ production in the diseased organ is associated with the disease process, as it was found only in the CNS and neither in the spleens of these mice (Fig. 1B) nor in CNS of mice that were not induced with EAE (Table 1). IL-10 has potent immunoregulatory properties and is selectively up-regulated in brain lesions during the recovery phase of EAE and MS (8–11). The reduction of INF-γ and the elevation of IL-10 are therefore of therapeutic significance. A Th1 to Th2 cytokine shift caused by Cop 1 treatment was previously shown both in EAE-induced mice (14, 15) and in MS patients (16–18). However, this effect was demonstrated in the periphery and not in the CNS. The present results are the first demonstration that this beneficial effect occurs in the diseased organ. Thus, a direct linkage can be drawn between the therapeutic activity of Cop 1 in EAE/MS and an in situ immunomodulatory effect, namely the appearance of Cop 1-specific Th2 cells, as well as Th1 to Th2 cytokine shift in response to the autoantigen MBP that is present in the CNS.
Acknowledgments
We thank Professors Irun R. Cohen, Reinhard Hohlfeld, and Michal Schwartz for critically reviewing this manuscript. This work was supported by a grant from Teva Pharmaceutical Industries, Ltd. (Israel).
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MBP
myelin basic protein
- Cop 1
copolymer 1
- Th1 and Th2
T helper types 1 and 2
- IFN
interferon
- CNS
central nervous system
- BBB
blood–brain barrier: TGF, transforming growth factor
References
- 1.Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 2.Cross A H, O'Mara T, Raine C S. Neurology. 1993;43:1028–1033. doi: 10.1212/wnl.43.5.1028. [DOI] [PubMed] [Google Scholar]
- 3.Wekerle H. Lab Invest. 1984;51:199–205. [PubMed] [Google Scholar]
- 4.Lehmann P V. Am J Pathol. 1998;153:677–680. doi: 10.1016/S0002-9440(10)65609-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teitelbaum D, Aharoni R, Fridkis-Hareli M, Arnon R, Sela M. In: The Decade in Autoimmunity. Shoenfeld Y, editor. New York: Elsevier; 1998. pp. 183–188. [Google Scholar]
- 6.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Sciffer R B, et al. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 7.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Sciffer R B, et al. Neurology. 1998;50:701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
- 8.Liblau R S, Singer S M, McDevitt H O. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 9.Adorini L, Sinigaglia F. Immunol Today. 1997;18:209–211. doi: 10.1016/s0167-5699(97)01031-1. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy M K, Torrance D S, Picha K S, Mohler K M. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 11.Tanuma N, Kojima T, Shin T, Aikawa Y, Kohji T, Ishihara Y, Matsumoto Y. J Neuroimmunol. 1997;73:197–206. doi: 10.1016/s0165-5728(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 12.Lando Z, Teitelbaum D, Arnon R. J Immunol. 1979;132:2156–2160. [PubMed] [Google Scholar]
- 13.Aharoni R, Teitelbaum D, Arnon R. Eur J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 14.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aharoni R, Teitelbaum D, Sela M, Arnon R. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 16.Miller A, Shapiro S, Gershtein R, Kinarti A, Rawashdeh H, Hongman S, Lahat N. J Neuroimmunol. 1998;92:113–121. doi: 10.1016/s0165-5728(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 17.Neuhaus O, Farina C, Yassouridis A, Wiendl H, Bergh F T, Dose T, Wekerle H, Hohlfeld R. Proc Natl Acad Sci USA. 2000;97:7452–7457. doi: 10.1073/pnas.97.13.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duda P W, Schmied M C, Cook S L, Krieger J I, Hafler D A. J Clin Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfeld H, Teitelbaum D, Arnon R, Sela M. FEBS Lett. 1970;7:317–320. doi: 10.1016/0014-5793(70)80193-4. [DOI] [PubMed] [Google Scholar]
- 21.D'Ambrosio D, Lellen A, Colantonio L, Clissi B, Pardi R, Sinigaglia F. Immunol Today. 2000;21:183–186. doi: 10.1016/s0167-5699(00)01590-5. [DOI] [PubMed] [Google Scholar]
- 22.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. Nature (London) 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 23.Lafaille J, Van de Keere F, Hsu A L, Baron J B, Hass W, Raine C S, Tonegawa S. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krakowski M L, Owens T. Eur J Immunol. 1997;27:2840–2847. doi: 10.1002/eji.1830271115. [DOI] [PubMed] [Google Scholar]
- 25.Ablamunits V, Elias D, Cohen I R. Clin Exp Immunol. 1999;115:260–267. doi: 10.1046/j.1365-2249.1999.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]