Abstract
AIMS—To assess the efficacy of isovolaemic haemodilution therapy (IHT) in the treatment of patients with branch retinal vein occlusion (BRVO). METHODS—Patients presenting with BRVO between 1 July 1991 and 31 August 1993 were eligible for inclusion and randomised into treatment and control groups. Patients randomised to receive IHT were treated for 6 weeks with venesection and volume replacement using hydroxyethylstarch, a plasma expander. The target haematocrit was 35%. Follow up was for 1 year. RESULTS—The baseline visual acuity of the two groups was similar at 0.74 and 0.75 logMAR units (Snellen 6/36), for the IHT and control groups, respectively. At 6 weeks, visual acuity in the IHT group had improved by 0.20 logMAR units (2 lines on the Bailey-Lovie chart) (p=0.0001). Vision was unchanged in the control group. At 1 year, the IHT group exhibited an improvement of 0.43 logMAR units. By comparison, the improvement in the control group at 1 year was significantly less at 0.17 logMAR units (p=0.03). The final visual acuity in the IHT and control groups was 0.30 (Snellen 6/12) and 0.60 (Snellen 6/24) logMAR units, respectively. CONCLUSIONS—The results support the theory that IHT has a positive effect on the visual outcome in patients with BRVO. Keywords: branch retinal vein occlusion; haemodilution; laser treatment; macular oedema
Full Text
The Full Text of this article is available as a PDF (131.0 KB).
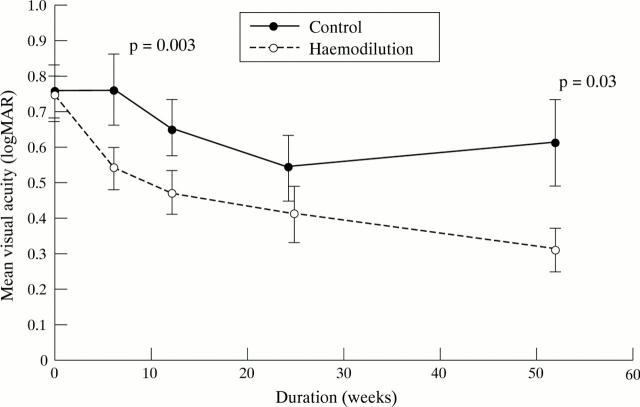
Figure 1 .
Mean visual acuity. Each point shows the mean visual acuity (vertical bars, mean (SEM)) in both groups of patients from entry into the study to 1 year. The visual acuity is presented in logMAR units; a decreasing value indicates an improving visual acuity. The p value at which the difference between the two groups reaches statistical significance is shown.
Figure 2 .
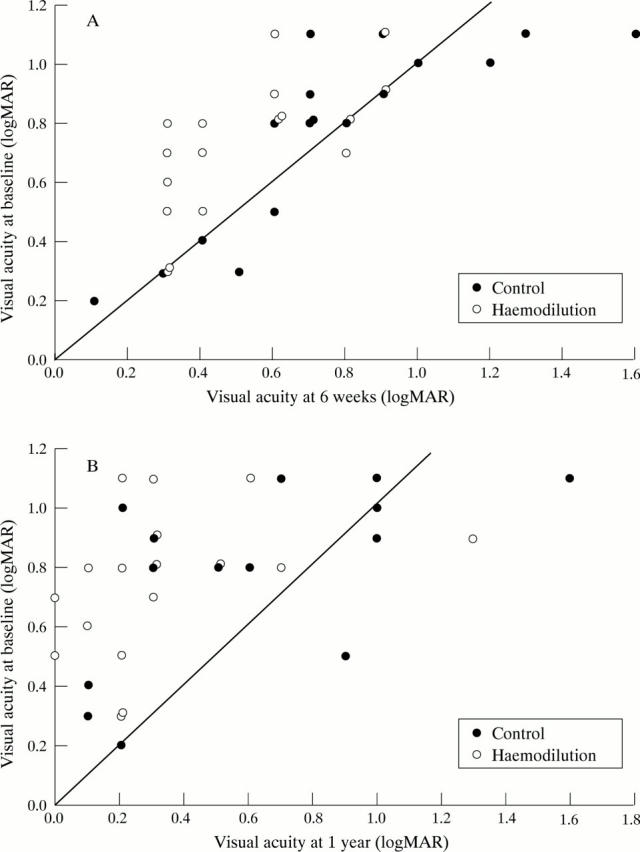
(A) Visual acuity at 6 weeks. This presents the visual acuity at entry into the study and at 6 weeks. Values on the diagonal line are those experiencing no change; those above the line improved and those below deteriorated. (B) Visual acuity at 1 year. This presents the visual acuity at entry into the study and at 1 year. Values on the diagonal line are those experiencing no change; those above the line improved and those below deteriorated.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton N. Pathophysiology of retinal cotton-wool spots. Br Med Bull. 1970 May;26(2):143–150. doi: 10.1093/oxfordjournals.bmb.a070766. [DOI] [PubMed] [Google Scholar]
- Chabanel A., Glacet-Bernard A., Lelong F., Taccoen A., Coscas G., Samama M. M. Increased red blood cell aggregation in retinal vein occlusion. Br J Haematol. 1990 May;75(1):127–131. doi: 10.1111/j.1365-2141.1990.tb02628.x. [DOI] [PubMed] [Google Scholar]
- Clemett R. S. Retinal branch vein occlusion. Changes at the site of obstruction. Br J Ophthalmol. 1974 May;58(5):548–554. doi: 10.1136/bjo.58.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell J. W., Smith E. E. Determinant of the optimal hematocrit. J Appl Physiol. 1967 Mar;22(3):501–504. doi: 10.1152/jappl.1967.22.3.501. [DOI] [PubMed] [Google Scholar]
- Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992 Oct;110(10):1427–1434. doi: 10.1001/archopht.1992.01080220089028. [DOI] [PubMed] [Google Scholar]
- Hansen L. L., Danisevskis P., Arntz H. R., Hövener G., Wiederholt M. A randomised prospective study on treatment of central retinal vein occlusion by isovolaemic haemodilution and photocoagulation. Br J Ophthalmol. 1985 Feb;69(2):108–116. doi: 10.1136/bjo.69.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. L., Wiek J., Schade M., Müller-Stolzenburg N., Wiederholt M. Effect and compatibility of isovolaemic haemodilution in the treatment of ischaemic and non-ischaemic central retinal vein occlusion. Ophthalmologica. 1989;199(2-3):90–99. doi: 10.1159/000310023. [DOI] [PubMed] [Google Scholar]
- Hansen L. L., Wiek J., Wiederholt M. A randomised prospective study of treatment of non-ischaemic central retinal vein occlusion by isovolaemic haemodilution. Br J Ophthalmol. 1989 Nov;73(11):895–899. doi: 10.1136/bjo.73.11.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley D. J., Tripathi R. C., Ashton N. Experimental retinal branch vein occlusion in the monkey. Histopathological and ultrastructural studies. Trans Ophthalmol Soc U K. 1976 Jul;96(2):202–209. [PubMed] [Google Scholar]
- Hulse J. D., Yacobi A. Hetastarch: an overview of the colloid and its metabolism. Drug Intell Clin Pharm. 1983 May;17(5):334–341. doi: 10.1177/106002808301700503. [DOI] [PubMed] [Google Scholar]
- Janvrin S. B., Davies G., Greenhalgh R. M. Postoperative deep vein thrombosis caused by intravenous fluids during surgery. Br J Surg. 1980 Oct;67(10):690–693. doi: 10.1002/bjs.1800671004. [DOI] [PubMed] [Google Scholar]
- Jung F., Koscielny J., Mrowietz C., Wolf S., Kiesewetter H., Wenzel E. Einfluss der Hämodilution auf den systemischen und den Kapillarhämatokrit. Infusionstherapie. 1990 Oct;17(5):268–275. [PubMed] [Google Scholar]
- Kiraly J. F., 3rd, Feldmann J. E., Wheby M. S. Hazards of phlebotomy in polycythemic patients with cardiovascular disease. JAMA. 1976 Nov 1;236(18):2080–2081. [PubMed] [Google Scholar]
- Kohner E. M., Dollery C. T., Shakib M., Henkind P., Paterson J. W., De Oliveira L. N., Bulpitt C. J. Experimental retinal branch vein occlusion. Am J Ophthalmol. 1970 May;69(5):778–825. doi: 10.1016/0002-9394(70)93420-3. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Firrell J. C. Microvascular hemodynamics during systemic hemodilution and hemoconcentration. Am J Physiol. 1986 Jun;250(6 Pt 2):H908–H922. doi: 10.1152/ajpheart.1986.250.6.H908. [DOI] [PubMed] [Google Scholar]
- McGrath M. A., Wechsler F., Hunyor A. B., Penny R. Systemic factors contributory to retinal vein occlusion. Arch Intern Med. 1978 Feb;138(2):216–220. [PubMed] [Google Scholar]
- Mirhashemi S., Ertefai S., Messmer K., Intaglietta M. Model analysis of the enhancement of tissue oxygenation by hemodilution due to increased microvascular flow velocity. Microvasc Res. 1987 Nov;34(3):290–301. doi: 10.1016/0026-2862(87)90062-8. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. The influence of pH and temperature on some physical properties of normal erythrocytes and erythrocytes from patients with hereditary spherocytosis. J Lab Clin Med. 1967 May;69(5):758–775. [PubMed] [Google Scholar]
- Orth D. H., Patz A. Retinal branch vein occlusion. Surv Ophthalmol. 1978 May-Jun;22(6):357–376. doi: 10.1016/0039-6257(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Peduzzi M., Debbia A., Guerrieri F., Bolzani R. Abnormal blood rheology in retinal vein occlusion. A preliminary report. Graefes Arch Clin Exp Ophthalmol. 1986;224(1):83–85. doi: 10.1007/BF02144143. [DOI] [PubMed] [Google Scholar]
- Pournaras C. J., Tsacopoulos M., Strommer K., Gilodi N., Leuenberger P. M. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990 Oct;97(10):1321–1328. doi: 10.1016/s0161-6420(90)32415-6. [DOI] [PubMed] [Google Scholar]
- Ring C. P., Pearson T. C., Sanders M. D., Wetherley-Mein G. Viscosity and retinal vein thrombosis. Br J Ophthalmol. 1976 Jun;60(6):397–410. doi: 10.1136/bjo.60.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf J., von Kummer R., Back T., Reich H., Machens G., Wildemann B. Haemodilution with dextran 40 and hydroxyethyl starch and its effect on cerebral microcirculation. J Neurol. 1989 Mar;236(3):164–167. doi: 10.1007/BF00314334. [DOI] [PubMed] [Google Scholar]
- Self F., McIntire L. V., Zanger B. Rheological evaluation of hemoglobin S and hemoglobin C hemoglobinopathies. J Lab Clin Med. 1977 Mar;89(3):488–497. [PubMed] [Google Scholar]
- St Louis P. J., Sulakhe P. V. Phosphorylation of cardiac sarcolemma by endogenous and exogenous protein kinases. Arch Biochem Biophys. 1979 Nov;198(1):227–240. doi: 10.1016/0003-9861(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. J. Whole blood viscosity and cerebral blood flow. Stroke. 1982 May-Jun;13(3):285–287. doi: 10.1161/01.str.13.3.285. [DOI] [PubMed] [Google Scholar]
- Trope G. E., Lowe G. D., McArdle B. M., Douglas J. T., Forbes C. D., Prentice C. M., Foulds W. S. Abnormal blood viscosity and haemostasis in long-standing retinal vein occlusion. Br J Ophthalmol. 1983 Mar;67(3):137–142. doi: 10.1136/bjo.67.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. O. Pathology of cystoid macular edema. Ophthalmology. 1982 Aug;89(8):902–915. doi: 10.1016/s0161-6420(82)34698-9. [DOI] [PubMed] [Google Scholar]
- Waters L. M., Christensen M. A., Sato R. M. Hetastarch: an alternative colloid in burn shock management. J Burn Care Rehabil. 1989 Jan-Feb;10(1):11–16. [PubMed] [Google Scholar]