Abstract
AIM—Orbital inflammation is common, but the mechanisms underlying leucocytic infiltration of orbital tissue are poorly understood. Human orbital fibroblasts (OF) express chemokines, interleukin 8 (IL-8) and monocyte chemotactic protein 1 (MCP-1), when exposed to proinflammatory cytokines. The effects of dexamethasone (DEX) and cyclosporin A (CSA) on OF IL-8 and MCP-1 were examined. METHODS—Cultured human OF were incubated with recombinant interleukin 1β (rIL-1β; 0.2, 2.0, 20 ng/ml) alone or incubated with rIL-1β and DEX (10-8, 10-7, 10-6 M) or CSA (3, 30, 300 ng/ml) for 24 hours. ELISA and northern blot analyses were performed to determine OF IL-8 and MCP-1 protein secretion and mRNA expression, respectively. RESULTS—OF lacked constitutive IL-8 or MCP-1 expression, but secreted significant amounts of these chemokines and expressed substantial steady state mRNA for both chemokines upon rIL-1β stimulation. DEX caused dose dependent inhibition of IL-1 induced IL-8 (p<0.001) and MCP-1 (p<0.05) secretion and mRNA expression at all concentrations of rIL-1β. CSA enhanced IL-1 induced OF IL-8 (p<0.001) and suppressed rIL-1β induced OF MCP-1 (p<0.05) secretion when lower doses of rIL-1β were used. These effects on secreted chemokines at different concentrations of rIL-1β and immunomodulating agents were corroborated by steady state OF IL-8 and MCP-1 mRNA expression. CONCLUSIONS—DEX is a potent inhibitor of OF IL-8 and MCP-1. In contrast, CSA enhances IL-1 induced OF IL-8 and suppresses OF MCP-1. These observations may explain the relative lack of CSA effectiveness in human orbital diseases that respond to corticosteroids. Keywords: orbital fibroblasts; chemokines; dexamethasone; cyclosporin A
Full Text
The Full Text of this article is available as a PDF (159.4 KB).
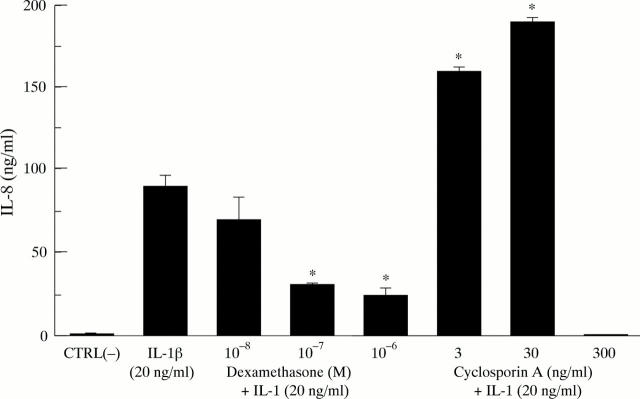
Figure 1 .
Dose dependent effects of dexamethasone and cyclosporin A on rIL-1β induced IL-8 secretion by orbital fibroblasts detected by ELISA. *Denotes p<0.001 compared with rIL-1β stimulated control.
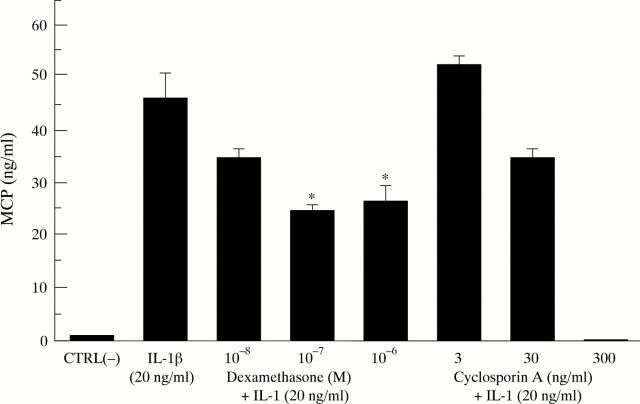
Figure 2 .
Dose dependent effects of dexamethasone and cyclosporin A on rIL-1β induced MCP-1 secretion by orbital fibroblasts detected by ELISA. *Denotes p<0.05 compared with rIL-1β stimulated control.
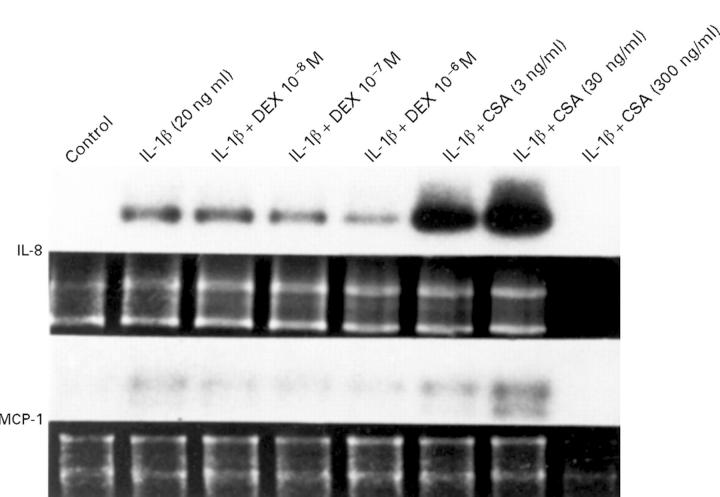
Figure 3 .
Representative northern blots revealing the effects of dexamethasone (DEX) and cyclosporin A (CSA) on rIL-1β induced (20 ng/ml) IL-8 and MCP-1 mRNA expression in human orbital fibroblasts at 24 hours. Equivalent total cellular RNA loading per lane is demonstrated by the electrophoretic profile (below) of 18s and 28s rRNA in all lanes, except the high dose CSA lane in which cell death occurred from cyclosporin toxicity.
Figure 4 .
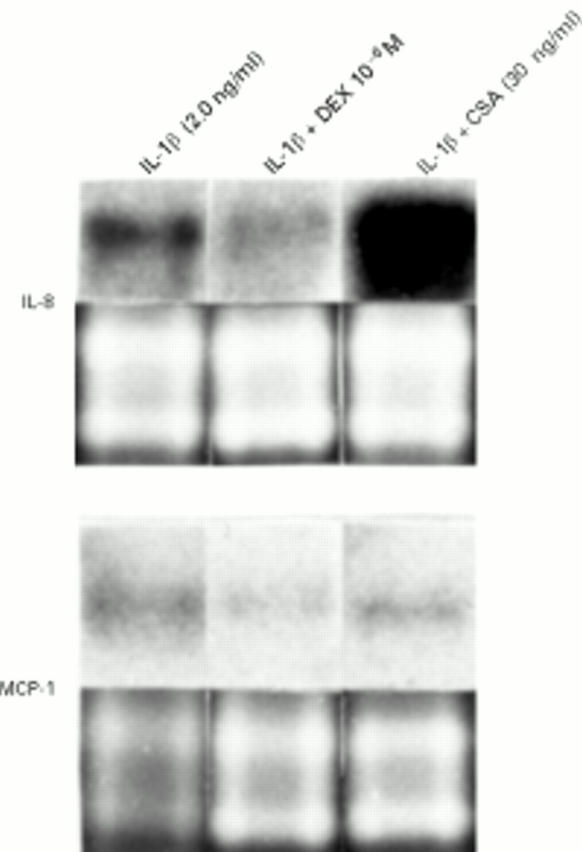
Representative blots revealing the effects of dexamethasone (DEX) and cyclosporin A (CSA) on OF IL-8 and MCP-1 mRNA expression in human orbital fibroblasts at 24 hours in response to rIL-1β OF stimulation (2.0 ng/ml). Equivalent total cellular RNA loading per lane is demonstrated by the electrophoretic profile (below) of 18s and 28s rRNA in all lanes. Similar results were found with rIL-1β (0.2 ng/ml) stimulation (data not shown).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu el-Asrar A. M., Van Damme J., Put W., Veckeneer M., Dralands L., Billiau A., Missotten L. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997 May;123(5):599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- Antony V. B., Hott J. W., Kunkel S. L., Godbey S. W., Burdick M. D., Strieter R. M. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol. 1995 Jun;12(6):581–588. doi: 10.1165/ajrcmb.12.6.7766422. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Bahn R. S., Garrity J. A., Gorman C. A. Clinical review 13: Diagnosis and management of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1990 Sep;71(3):559–563. doi: 10.1210/jcem-71-3-559. [DOI] [PubMed] [Google Scholar]
- Bahn R. S., Heufelder A. E. Retroocular fibroblasts: important effector cells in Graves' ophthalmopathy. Thyroid. 1992 Spring;2(1):89–94. doi: 10.1089/thy.1992.2.89. [DOI] [PubMed] [Google Scholar]
- Berkman N., Robichaud A., Krishnan V. L., Roesems G., Robbins R., Jose P. J., Barnes P. J., Chung K. F. Expression of RANTES in human airway epithelial cells: effect of corticosteroids and interleukin-4, -10 and -13. Immunology. 1996 Apr;87(4):599–603. doi: 10.1046/j.1365-2567.1996.477579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Jaffe G. J., Stuart A., Kunkel S. L., Strieter R. M. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995 Nov;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Strieter R. M., Elner V. M., Rollins B. J., Del Monte M. A., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991 Jun;64(6):819–825. [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Ferrick M. R., Thurau S. R., Oppenheim M. H., Herbort C. P., Ni M., Zachariae C. O., Matsushima K., Chan C. C. Ocular inflammation stimulated by intravitreal interleukin-8 and interleukin-1. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1534–1539. [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993 Jan;23(1):10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Elevated expression in situ of selectin and immunoglobulin superfamily type adhesion molecules in retroocular connective tissues from patients with Graves' ophthalmopathy. Clin Exp Immunol. 1993 Mar;91(3):381–389. doi: 10.1111/j.1365-2249.1993.tb05913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Graves' immunoglobulins and cytokines stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) in cultured Graves' orbital fibroblasts. Eur J Clin Invest. 1992 Aug;22(8):529–537. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Modulation of Graves' orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):120–127. [PubMed] [Google Scholar]
- Heufelder A. E., Smith T. J., Gorman C. A., Bahn R. S. Increased induction of HLA-DR by interferon-gamma in cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metab. 1991 Aug;73(2):307–313. doi: 10.1210/jcem-73-2-307. [DOI] [PubMed] [Google Scholar]
- Imam A. P., Halpern G. M. Uses, adverse effects of abuse of corticosteroids. Part I. Allergol Immunopathol (Madr) 1994 Nov-Dec;22(6):250–260. [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J. S., Dresner S. C. The nonspecific orbital inflammatory syndromes. Surv Ophthalmol. 1984 Sep-Oct;29(2):93–103. doi: 10.1016/0039-6257(84)90166-8. [DOI] [PubMed] [Google Scholar]
- Klett Z. G., Elner S. G., Elner V. M. Differential expression of immunoreactive HLA-DR and ICAM-1 in human cultured orbital fibroblasts and orbital tissue. Ophthal Plast Reconstr Surg. 1996 Sep;12(3):153–162. doi: 10.1097/00002341-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Korducki J. M., Loftus S. J., Bahn R. S. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest Ophthalmol Vis Sci. 1992 May;33(6):2037–2042. [PubMed] [Google Scholar]
- Nelson P. A., Kawamura A., Akselband Y., Peattie D. A., Aldape R. A., Harding M. W. Effect of immunosuppressive drugs on cytokine gene transcription studied by message amplification phenotyping (MAPPing) polymerase chain reaction. Transplant Proc. 1991 Dec;23(6):2867–2869. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Planck S. T., Huang X. N., Rich L., Ansel J. C. Detection of mRNA for the cytokines, interleukin-1 alpha and interleukin-8, in corneas from patients with pseudophakic bullous keratopathy. Invest Ophthalmol Vis Sci. 1995 Sep;36(10):2151–2155. [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A., Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991 May;72(5):1169–1171. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Koch A. E., Antony V. B., Fick R. B., Jr, Standiford T. J., Kunkel S. L. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J Lab Clin Med. 1994 Feb;123(2):183–197. [PubMed] [Google Scholar]
- Weetman A. P. Thyroid-associated eye disease: pathophysiology. Lancet. 1991 Jul 6;338(8758):25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- White V., Rootman J., Quenville N., Worth A., Robertson W. Orbital lymphoproliferative and inflammatory lesions. Can J Ophthalmol. 1987 Dec;22(7):362–373. [PubMed] [Google Scholar]
- Wiederrecht G., Lam E., Hung S., Martin M., Sigal N. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993 Nov 30;696:9–19. doi: 10.1111/j.1749-6632.1993.tb17137.x. [DOI] [PubMed] [Google Scholar]
- Wingett D., Forcier K., Nielson C. P. Glucocorticoid-mediated inhibition of RANTES expression in human T lymphocytes. FEBS Lett. 1996 Dec 2;398(2-3):308–311. doi: 10.1016/s0014-5793(96)01238-0. [DOI] [PubMed] [Google Scholar]