Abstract
AIMS/BACKGROUND—The purpose of this study was apply the polymerase chain reaction (PCR) to develop a sensitive, specific, and rapid test to diagnose Fusarium keratitis. Fusarium is the most common cause of fungal corneal infection in some parts of the world. It is often difficult to establish that a keratitis is due to fungal infection. METHODS—Fusarium solani keratitis was induced in three eyes of three rabbits by injection of a suspension of the fungus into the anterior corneal stroma. In one rabbit the contralateral eye served as a control. From four to 28 days after inoculation, the corneas were scraped for culture, then scraped and swabbed for PCR analysis. The PCR was performed with primers directed against a portion of the Fusarium cutinase gene, and the presence or absence of this amplified target sequence was determined by agarose gel. RESULTS—The amplified DNA sequence was detected in 25 of 28 samples from the corneas infected with Fusarium, for a sensitivity of 89%. Only three of the 14 samples from these eyes with Fusarium keratitis were positive by culture, for a sensitivity of 21%. Seven of eight control samples were negative by the PCR based test, for a specificity of 88%. CONCLUSION—This PCR based test holds promise of being an effective method of diagnosing Fusarium keratitis as well as Fusarium infections at other sites. Keywords: keratitis; Fusarium; ulcer; cornea; polymerase chain reaction
Full Text
The Full Text of this article is available as a PDF (163.5 KB).
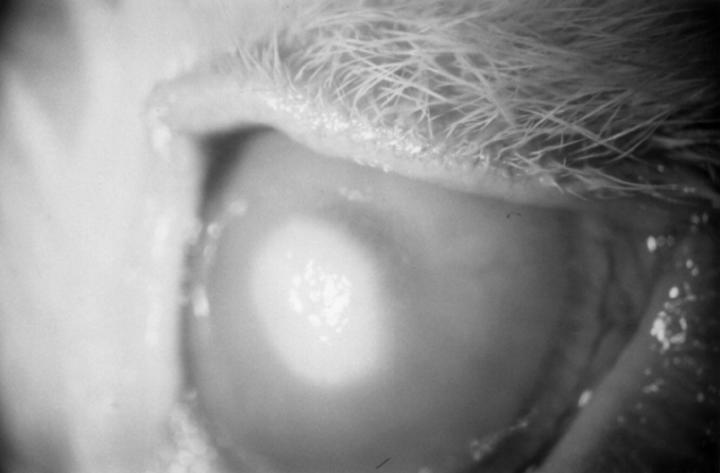
Figure 1 .
A rabbit cornea 5 days after inoculation with Fusarium solani, showing a focal infiltrate and diffuse corneal oedema.
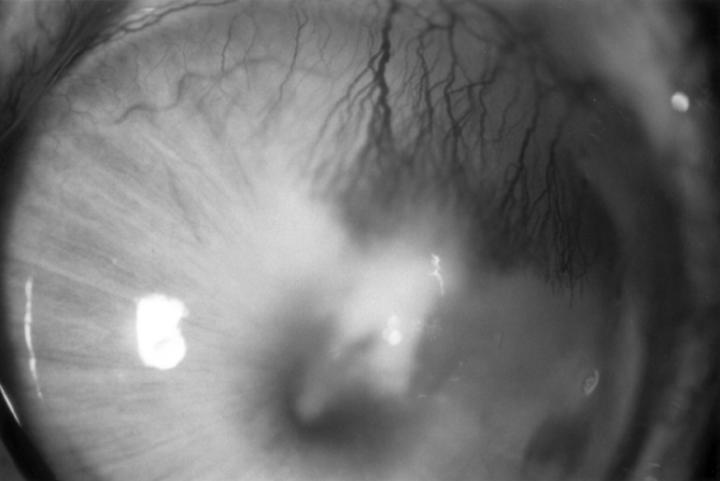
Figure 2 .
A rabbit cornea 12 days after inoculation with Fusarium solani, showing vascularisation of the infiltrate.
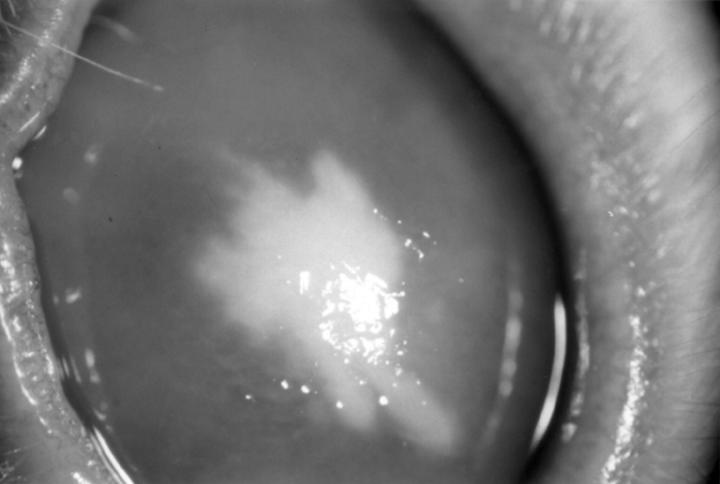
Figure 3 .
A rabbit cornea 4 days after inoculation with Candida albicans, showing a focal infiltrate and diffuse corneal oedema.
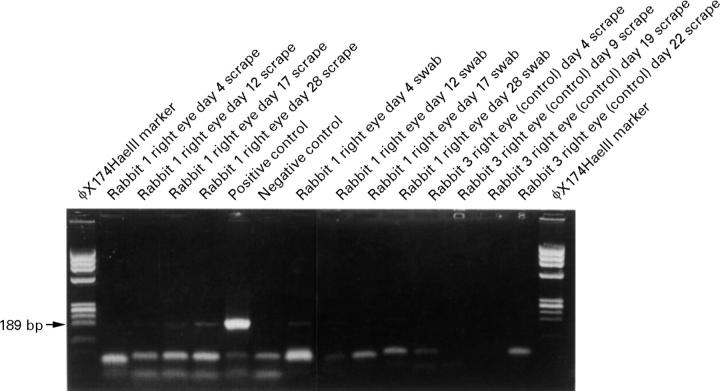
Figure 4 .
Agarose gel showing PCR results of samples collected by scraping and swabbing Fusarium infected and control corneas. Many of the positive results from the Fusarium infected eyes consist of only faint bands of the target fragment of the cutinase gene, which are lost on reproduction of the photograph. However, the faint bands were confirmed by Southern blot analysis. As expected, the PCR products from the control eye contained no detectable target DNA. A separate negative control was from the PCR performed at the same time with sterile water substituted for the sample. The positive control was PCR product from purified DNA extracted from fresh Fusarium mycelium.
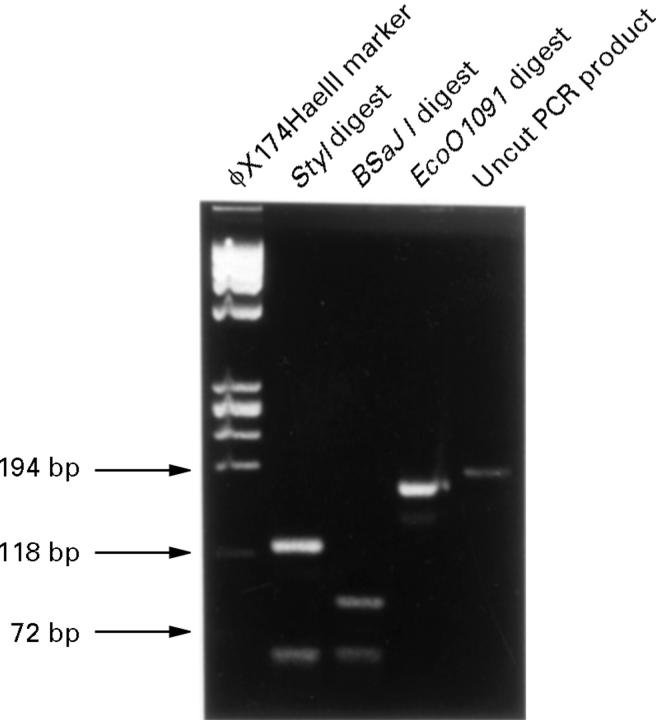
Figure 5 .
Agarose gel of restriction endonuclease digests of the PCR product. Fragments of the expected sizes were produced (Sty1: 122 base pair (bp) and 67 bp; BsaJ1: 87 bp, 67 bp, and 35 bp; EcoO109I: 174 bp and 15 bp), confirming that the product was the target fragment of the Fusarium cutinase gene.
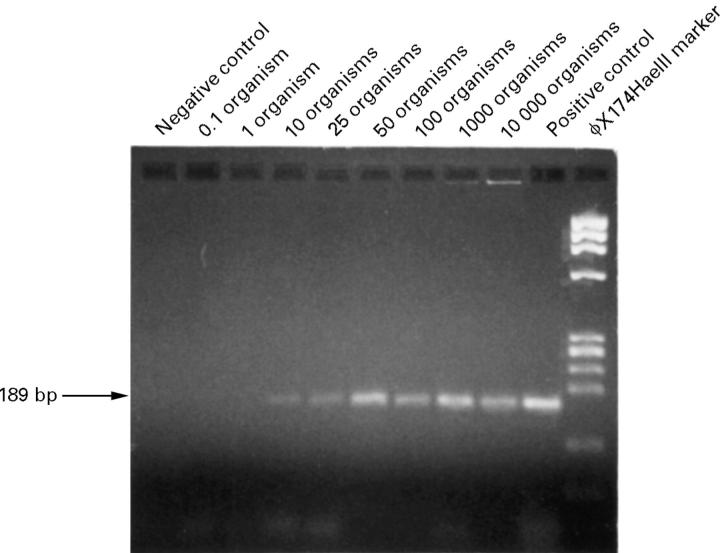
Figure 6 .
Agarose gel of PCR products from a serial dilution of Fusarium solani spores, demonstrating that this PCR based test can detect from 10 to 10 000 organisms in a sample. Positive and negative controls are as described in Figure 4.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrakis G., Sears M., Gloor P. Postmortem diagnosis of Fusarium panophthalmitis by the polymerase chain reaction. Am J Ophthalmol. 1996 Feb;121(2):221–223. doi: 10.1016/s0002-9394(14)70594-x. [DOI] [PubMed] [Google Scholar]
- Anaissie E., Kantarjian H., Ro J., Hopfer R., Rolston K., Fainstein V., Bodey G. The emerging role of Fusarium infections in patients with cancer. Medicine (Baltimore) 1988 Mar;67(2):77–83. doi: 10.1097/00005792-198803000-00001. [DOI] [PubMed] [Google Scholar]
- Aouizerate F., Cazenave J., Poirier L., Verin P., Cheyrou A., Begueret J., Lagoutte F. Detection of Toxoplasma gondii in aqueous humour by the polymerase chain reaction. Br J Ophthalmol. 1993 Feb;77(2):107–109. doi: 10.1136/bjo.77.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell P., Stenson S. Ulcerative keratitis. Survey of 30 years' laboratory experience. Arch Ophthalmol. 1982 Jan;100(1):77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- Cantin E., Chen J., Willey D. E., Taylor J. L., O'Brien W. J. Persistence of herpes simplex virus DNA in rabbit corneal cells. Invest Ophthalmol Vis Sci. 1992 Jul;33(8):2470–2475. [PubMed] [Google Scholar]
- Cho C. T., Vats T. S., Lowman J. T., Brandsberg J. W., Tosh F. E. Fusarium solani infection during treatment for acute leukemia. J Pediatr. 1973 Dec;83(6):1028–1031. doi: 10.1016/s0022-3476(73)80543-8. [DOI] [PubMed] [Google Scholar]
- Forster R. K., Rebell G. Animal model of Fusarium solani keratitis. Am J Ophthalmol. 1975 Mar;79(3):510–515. doi: 10.1016/0002-9394(75)90629-7. [DOI] [PubMed] [Google Scholar]
- Forster R. K., Rebell G. The diagnosis and management of keratomycoses. I. Cause and diagnosis. Arch Ophthalmol. 1975 Oct;93(10):975–978. doi: 10.1001/archopht.1975.01010020769005. [DOI] [PubMed] [Google Scholar]
- Fox G. M., Crouse C. A., Chuang E. L., Pflugfelder S. C., Cleary T. J., Nelson S. J., Atherton S. S. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol. 1991 Feb;109(2):266–271. doi: 10.1001/archopht.1991.01080020112054. [DOI] [PubMed] [Google Scholar]
- Gugnani H. C., Talwar R. S., Njoku-Obi A. N., Kodilinye H. C. Mycotic keratitis in Nigeria. A study of 21 cases. Br J Ophthalmol. 1976 Sep;60(9):607–613. doi: 10.1136/bjo.60.9.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns K. J., O'Day D. M. Pharmacologic management of keratomycoses. Surv Ophthalmol. 1988 Nov-Dec;33(3):178–188. doi: 10.1016/0039-6257(88)90086-0. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Sexton R., Rebell G. Mycotic keratitis in South Florida: a review of thirty-nine cases. Trans Ophthalmol Soc U K. 1970;89:781–797. [PubMed] [Google Scholar]
- Jones D. B., Wilson L., Sexton R., Rebell G. Early diagnosis of mycotic keratitis. Trans Ophthalmol Soc U K. 1970;89:805–813. [PubMed] [Google Scholar]
- Kaye S. B., Lynas C., Patterson A., Risk J. M., McCarthy K., Hart C. A. Evidence for herpes simplex viral latency in the human cornea. Br J Ophthalmol. 1991 Apr;75(4):195–200. doi: 10.1136/bjo.75.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Liesegang T. J., Forster R. K. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980 Jul;90(1):38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- McDonnell J. M., McDonnell P. J., Sun Y. Y. Human papillomavirus DNA in tissues and ocular surface swabs of patients with conjunctival epithelial neoplasia. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):184–189. [PubMed] [Google Scholar]
- McDonnell P. J., Nobe J., Gauderman W. J., Lee P., Aiello A., Trousdale M. Community care of corneal ulcers. Am J Ophthalmol. 1992 Nov 15;114(5):531–538. doi: 10.1016/s0002-9394(14)74479-4. [DOI] [PubMed] [Google Scholar]
- Minor R. L., Jr, Pfaller M. A., Gingrich R. D., Burns L. J. Disseminated Fusarium infections in patients following bone marrow transplantation. Bone Marrow Transplant. 1989 Nov;4(6):653–658. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nishi M., Hanashiro R., Mori S., Masuda K., Mochizuki M., Hondo R. Polymerase chain reaction for the detection of the varicella-zoster genome in ocular samples from patients with acute retinal necrosis. Am J Ophthalmol. 1992 Nov 15;114(5):603–609. doi: 10.1016/s0002-9394(14)74491-5. [DOI] [PubMed] [Google Scholar]
- O'Day D. M., Akrabawi P. L., Head W. S., Ratner H. B. Laboratory isolation techniques in human and experimental fungal infections. Am J Ophthalmol. 1979 May;87(5):688–693. doi: 10.1016/0002-9394(79)90305-2. [DOI] [PubMed] [Google Scholar]
- O'Day D. M., Ray W. A., Head W. S., Robinson R. D., Williams T. E. Influence of corticosteroid on experimentally induced keratomycosis. Arch Ophthalmol. 1991 Nov;109(11):1601–1604. doi: 10.1001/archopht.1991.01080110139051. [DOI] [PubMed] [Google Scholar]
- O'Day D. M. Selection of appropriate antifungal therapy. Cornea. 1987;6(4):238–245. doi: 10.1097/00003226-198706040-00002. [DOI] [PubMed] [Google Scholar]
- Polack F. M., Kaufman H. E., Newmark E. Keratomycosis. Medical and surgical treatment. Arch Ophthalmol. 1971 Apr;85(4):410–416. doi: 10.1001/archopht.1971.00990050412003. [DOI] [PubMed] [Google Scholar]
- Richardson S. E., Bannatyne R. M., Summerbell R. C., Milliken J., Gold R., Weitzman S. S. Disseminated fusarial infection in the immunocompromised host. Rev Infect Dis. 1988 Nov-Dec;10(6):1171–1181. doi: 10.1093/clinids/10.6.1171. [DOI] [PubMed] [Google Scholar]
- Rosa R. H., Jr, Miller D., Alfonso E. C. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994 Jun;101(6):1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Dickman M. B., Kolattukudy P. E. Structure of the cutinase gene and detection of promoter activity in the 5'-flanking region by fungal transformation. J Bacteriol. 1989 Apr;171(4):1942–1951. doi: 10.1128/jb.171.4.1942-1951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Flurkey W. H., Okita T. W., Kolattukudy P. E. Cloning and structure determination of cDNA for cutinase, an enzyme involved in fungal penetration of plants. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3939–3943. doi: 10.1073/pnas.81.13.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Su C. S., Bowden S., Fong L. P., Taylor H. R. Detection of hepatitis B virus DNA in tears by polymerase chain reaction. Arch Ophthalmol. 1994 May;112(5):621–625. doi: 10.1001/archopht.1994.01090170065024. [DOI] [PubMed] [Google Scholar]
- Talley A. R., Garcia-Ferrer F., Laycock K. A., Loeffelholz M., Pepose J. S. The use of polymerase chain reaction for the detection of chlamydial keratoconjunctivitis. Am J Ophthalmol. 1992 Dec 15;114(6):685–692. doi: 10.1016/s0002-9394(14)74045-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shimomura Y., Kinoshita S., Nishida K., Yamamoto R., Tano Y. Detection of herpes simplex virus DNA in human tear film by the polymerase chain reaction. Am J Ophthalmol. 1994 Feb 15;117(2):160–163. doi: 10.1016/s0002-9394(14)73071-5. [DOI] [PubMed] [Google Scholar]
- Yu D. D., Lemp M. A., Mathers W. D., Espy M., White T. Detection of varicella-zoster virus DNA in disciform keratitis using polymerase chain reaction. Arch Ophthalmol. 1993 Feb;111(2):167–168. doi: 10.1001/archopht.1993.01090020021010. [DOI] [PubMed] [Google Scholar]