Abstract
AIMS/BACKGROUND—There are more reagents and information available for immunological studies in the mouse compared with other animals. Unfortunately, the mouse penetrating keratoplasty model is associated with high background inflammation which hinders study of the immune response to the graft. To mitigate this drawback, a murine orthotopic corneal interlamellar transplantation model with mild non-specific inflammation was developed. METHODS—A 1.5 mm diameter full thickness donor corneal button was placed in a 2 mm diameter recipient corneal interlamellar pocket without placement of a suture. The clinical course of graft status was studied daily for 60 days in 30 allografts (donor strain CBA 101 (H-2k) to recipient NIH (H-2q)) and 30 syngeneic grafts (NIH to NIH) by slit lamp biomicroscopy and scored for neovascularisation, opacity, oedema, and granularity. In another cohort of animals, histological observation was performed after 30 minutes and on days 10, 20, 30, and 40 after transplantation (four allografts and four syngeneic grafts per time point). Histological study was also performed on grafts without donor epithelium and on interlamellar pockets without grafts. RESULTS—There was significantly more neovascularisation (NV), opacity, oedema, and granularity in 24/30 allografts (80%) than in syngeneic grafts. Such grafts were defined as rejected. The median time to rejection was 21 days (range 18 to >60 days). By histology, some allografts showed moderate to heavy cell infiltration which correlated with clinical scores of NV (4-5), opacity (1-3), oedema (1-3), and granularity (1-3). Such infiltration was absent in other allografts and syngeneic grafts. CONCLUSION—Surgically, corneal interlamellar transplantation could be accomplished in the mouse and rejection could be clearly defined. The model can therefore be useful for in situ study of cell and molecular aspects of corneal graft rejection. Keywords: interlamellar keratoplasty; corneal transplantation; graft rejection; mouse
Full Text
The Full Text of this article is available as a PDF (172.4 KB).
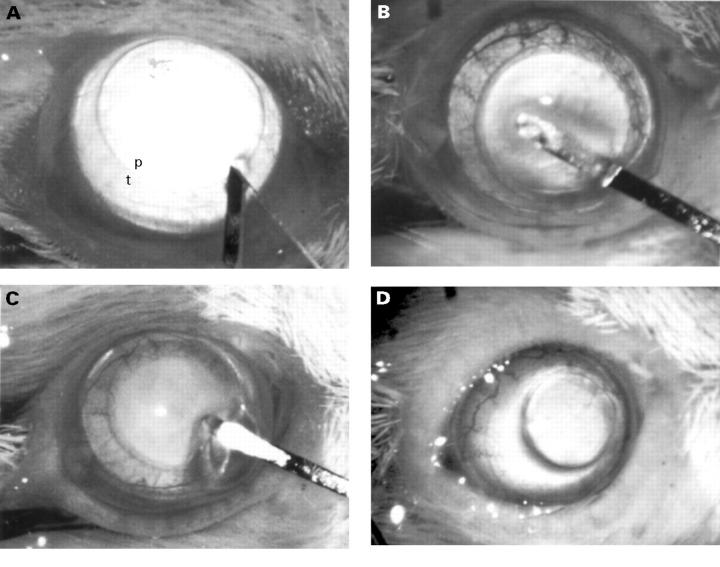
Figure 1 .
Transplantation procedure. (A) Cornea was marked with a 2 mm trephine, a small incision was made inferonasally, p = pupil margin, t = trephine mark. (B) An interlamellar tunnel was fashioned with a microblade, followed by interlamellar dissection. (C) After enlarging the incision with scissors from 3 to 6 o'clock, a 1.5 mm full thickness donor button was introduced into the pocket. (D) The graft remained in the pocket without use of a suture.
Figure 2 .
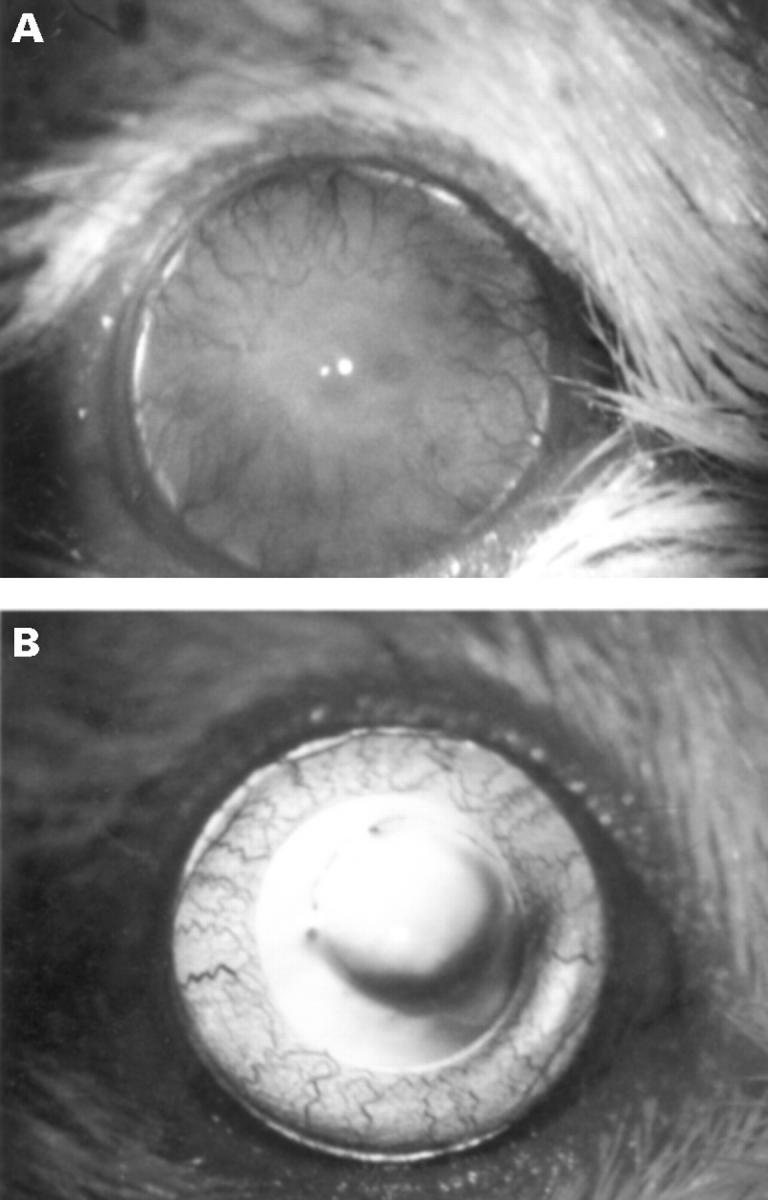
(A) An allograft undergoing rejection on day 25 after transplantation (neovascularisation, 4; opacity, 2; oedema, 1; granularity, 1). There is diffuse milky opacity of the graft obscuring the pupil, and heavy corneal neovascularisation. (B) A clear syngeneic graft, on day 25 after transplantation. There was no neovascularisation.
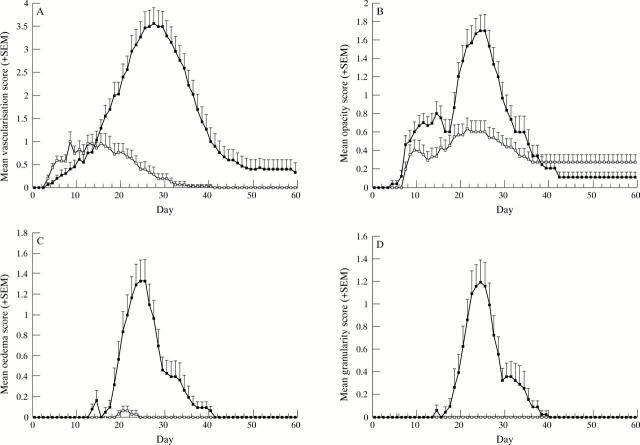
Figure 3 .
Clinical course of graft status. (A) Neovascularisation score (+SEM) (p <0.05 on days 4-6 and 16-41). (B) Opacity score (+SEM) (p <0.05 on days 11-12, 14, and 19-29). (C) Oedema score (+SEM) (p <0.05 on days 19-32). (D) Granularity score (+SEM) (p <0.05 on days 20-31). Solid squares represent allografts, and open squares represent syngeneic grafts.
Figure 4 .
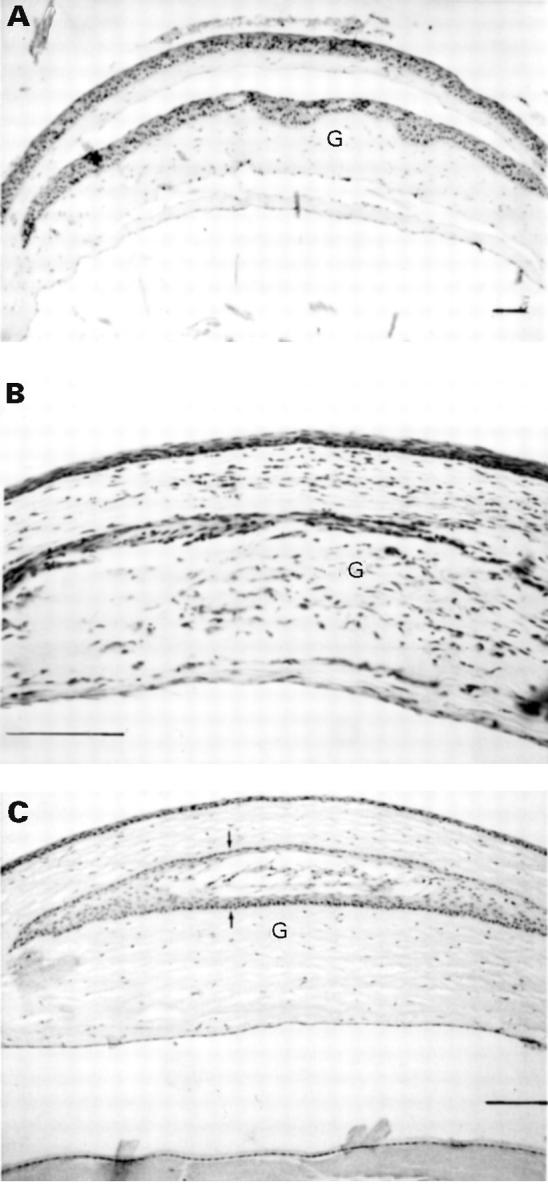
Histological appearance of grafted corneas. G = graft. (A) 30 minutes after transplantation; there is obvious separation between recipient and graft above and below. (B) 30 days after transplantation; an allograft showing dense cell infiltration with disappearance of some of the donor epithelium. (C) 30 days after transplantation; an intact syngeneic graft except for disappearance of donor endothelium. There is a fully developed epithelial cyst (arrows) but no cell infiltration. (Haematoxylin and eosin, bar represents 200 µm.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alldredge O. C., Krachmer J. H. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981 Apr;99(4):599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- Chandler J. W., Ray-Keil L., Gillette T. E. Experimental corneal allograft rejection: description of murine model and a new hypothesis of immunopathogenesis. Curr Eye Res. 1982;2(6):387–397. doi: 10.3109/02713688209000784. [DOI] [PubMed] [Google Scholar]
- Driebe W. T., Jr, Park J. Y., Meisler D. M. Epithelial rejection rings. Arch Ophthalmol. 1997 Jul;115(7):938–939. doi: 10.1001/archopht.1997.01100160108027. [DOI] [PubMed] [Google Scholar]
- Gronemeyer U. Methode der Interlamellären Keratoplastik am Rattenauge. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;185(1):75–82. doi: 10.1007/BF00410507. [DOI] [PubMed] [Google Scholar]
- Gronemeyer U., Pülhorn G., Müller-Ruchholtz W. Allogeneic corneal grafting in inbred strains of rats. Histology of graft reaction. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978 Nov 30;208(4):247–262. doi: 10.1007/BF00419380. [DOI] [PubMed] [Google Scholar]
- Joo C. K., Pepose J. S., Stuart P. M. T-cell mediated responses in a murine model of orthotopic corneal transplantation. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1530–1540. [PubMed] [Google Scholar]
- Khodadoust A. A. Penetrating keratoplasty in the rabbit. Am J Ophthalmol. 1968 Nov;66(5):899–905. doi: 10.1016/0002-9394(68)92809-2. [DOI] [PubMed] [Google Scholar]
- MAUMENEE A. E. The influence of donor-recipient sensitization on corneal grafts. Am J Ophthalmol. 1951 May;34(5 2):142–152. doi: 10.1016/0002-9394(51)90019-0. [DOI] [PubMed] [Google Scholar]
- Moticka E. J., She S. C. Comparison of failure rates of orthotopic corneal grafts using three different grafting procedures. Curr Eye Res. 1989 Aug;8(8):813–820. doi: 10.3109/02713688909000871. [DOI] [PubMed] [Google Scholar]
- Pleyer U., Steuhl K. P., Weidle E. G., Lisch W., Thiel H. J. Corneal graft rejection: incidence, manifestation, and interaction of clinical subtypes. Transplant Proc. 1992 Oct;24(5):2034–2037. [PubMed] [Google Scholar]
- Rinne J. R., Stulting R. D. Current practices in the prevention and treatment of corneal graft rejection. Cornea. 1992 Jul;11(4):326–328. doi: 10.1097/00003226-199207000-00010. [DOI] [PubMed] [Google Scholar]
- She S. C., Steahly L. P., Moticka E. J. A method for performing full-thickness, orthotopic, penetrating keratoplasty in the mouse. Ophthalmic Surg. 1990 Nov;21(11):781–785. [PubMed] [Google Scholar]
- Sonoda Y., Streilein J. W. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992 Oct;54(4):694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., McCulley J., Niederkorn J. Y. Heterotopic corneal grafting in mice: a new approach to the study of corneal alloimmunity. Invest Ophthalmol Vis Sci. 1982 Oct;23(4):489–500. [PubMed] [Google Scholar]
- Stulting R. D., Waring G. O., 3rd, Bridges W. Z., Cavanagh H. D. Effect of donor epithelium on corneal transplant survival. Ophthalmology. 1988 Jun;95(6):803–812. doi: 10.1016/s0161-6420(88)33120-9. [DOI] [PubMed] [Google Scholar]
- Tuberville A. W., Foster C. S., Wood T. O. The effect of donor cornea epithelium removal on the incidence of allograft rejection reactions. Ophthalmology. 1983 Nov;90(11):1351–1356. doi: 10.1016/s0161-6420(83)34395-5. [DOI] [PubMed] [Google Scholar]
- Vail A., Gore S. M., Bradley B. A., Easty D. L., Rogers C. A., Armitate W. J. Clinical and surgical factors influencing corneal graft survival, visual acuity, and astigmatism. Corneal Transplant Follow-up Study Collaborators. Ophthalmology. 1996 Jan;103(1):41–49. doi: 10.1016/s0161-6420(96)30734-3. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Coster D. J. Penetrating corneal transplantation in the inbred rat: a new model. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):23–30. [PubMed] [Google Scholar]
- Wilson S. E., Kaufman H. E. Graft failure after penetrating keratoplasty. Surv Ophthalmol. 1990 Mar-Apr;34(5):325–356. doi: 10.1016/0039-6257(90)90110-h. [DOI] [PubMed] [Google Scholar]
- Yao Y. F., Inoue Y., Miyazaki D., Shimomura Y., Ohashi Y., Tano Y. Ocular resurfacing and alloepithelial rejection in a murine keratoepithelioplasty model. Invest Ophthalmol Vis Sci. 1995 Dec;36(13):2623–2633. [PubMed] [Google Scholar]