Abstract
AIMS/BACKGROUND—Antimetabolites are increasingly used to manipulate the healing response after filtration surgery, but problems with thin cystic blebs have been encountered with the liquid agents commonly used such as 5-fluorouracil and mitomycin C. β Radiation appears to be a useful adjuvant treatment for preventing scarring after trabeculectomy, resulting in diffuse rather than cystic bleb formation, but much of the basic cell biology of the ocular fibroblast response to β radiation remains unclear. The effects of β radiation on ocular fibroblast proliferation and cell cycling were investigated to determine the nature and duration of these effects on these cells. METHODS—In vitro cell culture techniques were used to investigate fibroblast proliferation. Cell viability was studied using trypan blue dye exclusion. The effect of radiation on cell cycling was investigated using bromodeoxyuridine uptake. p53 expression was demonstrated using immunocytochemistry . RESULTS—β Radiation inhibited fibroblast proliferation in a dose dependent manner. Early cell death was not a prominent feature, but irradiated fibroblasts demonstrated a rapid onset and sustained period of growth arrest. p53 expression was found to be increased in irradiated cells. CONCLUSIONS—Single doses of β radiation significantly inhibit Tenon's capsule fibroblast proliferation in vitro over a 28 day period. This inhibition is the result of a rapid onset and sustained period of growth arrest in irradiated cells. Irradiated fibroblasts show an increase in p53 expression, a nuclear phosphoprotein which has been associated with control of the cell cycle. Single applications of β radiation may be an effective treatment for the prevention of bleb failure as a result of prolonged growth arrest of Tenon's capsule fibroblasts. Keywords: radiation; fibroblasts; growth arrest
Full Text
The Full Text of this article is available as a PDF (155.2 KB).
Figure 1 .
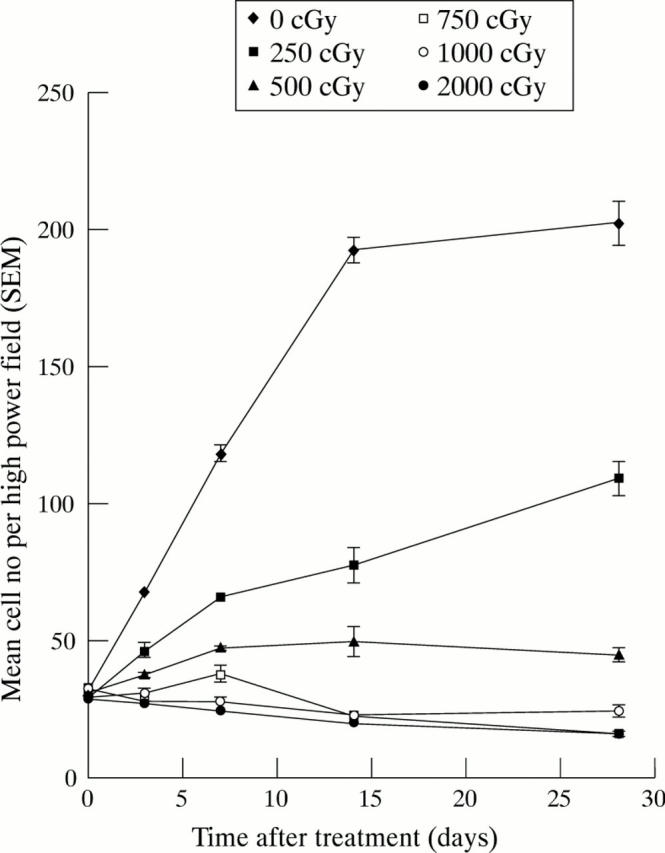
Effects of β radiation on fibroblast proliferation. Fibroblasts were grown in tissue culture, passaged, and used for proliferation experiments when in exponential phase growth. The effect of single doses of β radiation between 250 and 2000 cGy was investigated with control cells remaining unirradiated. At each time point, the mean number of human Tenon's fibroblasts (HTFs) in triplicate wells was determined for each dose of radiation studied. Single doses of β radiation significantly inhibited HTF proliferation relative to control cells at all time points (p<0.05 at each time point using one way ANOVA with Bonferroni's modification).
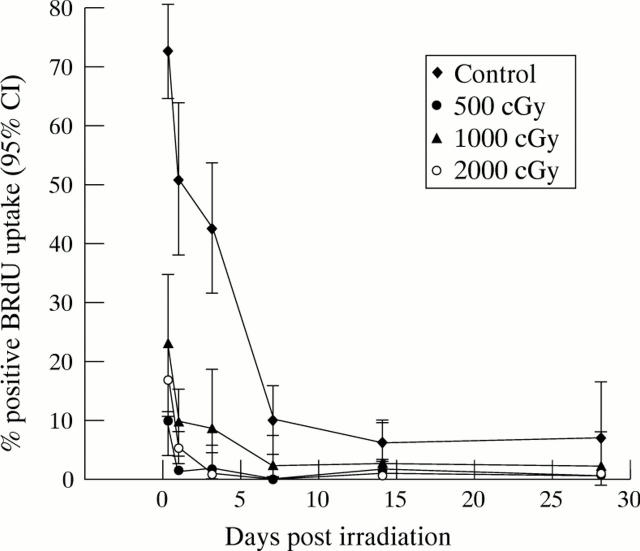
Figure 2 .
Cell cycling following β irradiation. Fibroblasts were cast into 96 well plates and allowed to settle overnight. Test wells were irradiated with 500, 1000, or 2000 cGy, and control cells remained unirradiated. At various time points following treatment, triplicate wells from each treatment group were pulsed in 1:1000 BRdU in DMEM/10% FCS for 24 hours, following which the incorporated BRdU was demonstrated by immunochemical antibody staining and DAB precipitation. The mean percentage number of labelled cells in each treatment group, together with the 95% confidence interval, was plotted at the various time points. Statistical analysis performed using a one way ANOVA test with Bonferroni's modification demonstrated that fibroblast proliferation was significantly greater in the control group than in all irradiated populations at all time points (p<0.05).
Figure 3 .
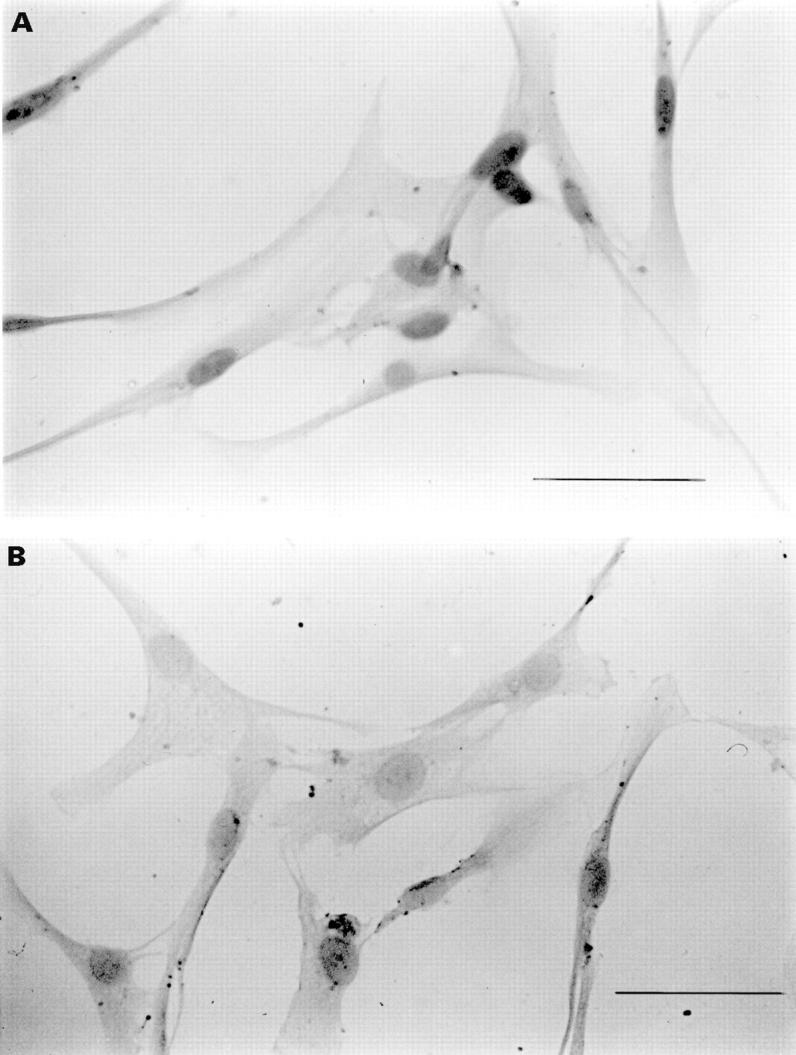
The effect of β radiation on p53 expression. (A) Irradiated fibroblasts at 24 hours (1000 cGy). Dark staining nuclei indicate positive p53 staining. (B) Unirradiated control fibroblasts at 24 hours. Bars = 50 µm.
Figure 4 .
Effects of β radiation on p53 expression. Monolayers of exponentially growing fibroblasts were prepared and treated as before. At each time point triplicate wells from each treatment group and control groups were fixed in 50:50 acetone/ethanol and then immunostained with Dako DO-7 anti-p53 antibody. Bound antibody was visualised using DAB immunoprecipitation and the percentage of positively staining cells was determined for each treatment and plotted against time.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. L., Agarwal A., Taylor W. R., Stark G. R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad M. I., Baum J. Optimal time for postoperative irradiation of pterygia. Ophthalmology. 1987 Nov;94(11):1450–1451. doi: 10.1016/s0161-6420(87)33267-1. [DOI] [PubMed] [Google Scholar]
- COHEN L. B., GRAHAM T. F., FRY W. E. Beta radiation; as an adjunct to glaucoma surgery in the Negro. Am J Ophthalmol. 1959 Jan;47(1 Pt 1):54–61. [PubMed] [Google Scholar]
- Cameron M. E. Beta irradiation as an adjunct to surgery in refractory glaucoma. Trans Aust Coll Ophthalmol. 1970;2:53–60. [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Cooper J. S. Postoperative irradiation of pterygia: ten more years of experience. Radiology. 1978 Sep;128(3):753–756. doi: 10.1148/128.3.753. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Clarkin K., Wahl G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994 Nov 1;8(21):2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Enoch T., Norbury C. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem Sci. 1995 Oct;20(10):426–430. doi: 10.1016/s0968-0004(00)89093-3. [DOI] [PubMed] [Google Scholar]
- Franks W. A., Hitchings R. A. Complications of 5--fluorouracil after trabeculectomy. Eye (Lond) 1991;5(Pt 4):385–389. doi: 10.1038/eye.1991.63. [DOI] [PubMed] [Google Scholar]
- Fuks Z., Persaud R. S., Alfieri A., McLoughlin M., Ehleiter D., Schwartz J. L., Seddon A. P., Cordon-Cardo C., Haimovitz-Friedman A. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994 May 15;54(10):2582–2590. [PubMed] [Google Scholar]
- Gratzner H. G., Leif R. C., Ingram D. J., Castro A. The use of antibody specific for bromodeoxyuridine for the immunofluorescent determination of DNA replication in single cells and chromosomes. Exp Cell Res. 1975 Oct 1;95(1):88–94. doi: 10.1016/0014-4827(75)90612-6. [DOI] [PubMed] [Google Scholar]
- Higginbotham E. J., Stevens R. K., Musch D. C., Karp K. O., Lichter P. R., Bergstrom T. J., Skuta G. L. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996 Apr;103(4):650–656. doi: 10.1016/s0161-6420(96)30639-8. [DOI] [PubMed] [Google Scholar]
- Hooper M. L. The role of the p53 and Rb-1 genes in cancer, development and apoptosis. J Cell Sci Suppl. 1994;18:13–17. doi: 10.1242/jcs.1994.supplement_18.3. [DOI] [PubMed] [Google Scholar]
- Jampel H. D., Pasquale L. R., Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin C. Arch Ophthalmol. 1992 Aug;110(8):1049–1050. doi: 10.1001/archopht.1992.01080200029011. [DOI] [PubMed] [Google Scholar]
- Joseph J. P., Grierson I., Hitchings R. A. Normal rabbit aqueous humour, fibronectin, and fibroblast conditioned medium are chemoattractant to Tenon's capsule fibroblasts. Eye (Lond) 1987;1(Pt 5):585–592. doi: 10.1038/eye.1987.90. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Katz G. J., Higginbotham E. J., Lichter P. R., Skuta G. L., Musch D. C., Bergstrom T. J., Johnson A. T. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology. 1995 Sep;102(9):1263–1269. doi: 10.1016/s0161-6420(95)30875-5. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Ward S., Grierson I., Rice N. S. Effect of beta radiation on proliferating human Tenon's capsule fibroblasts. Br J Ophthalmol. 1991 Oct;75(10):580–583. doi: 10.1136/bjo.75.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw P. T., Ward S., Porter A., Grierson I., Hitchings R. A., Rice N. S. The long-term effects of 5-fluorouracil and sodium butyrate on human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 1992 May;33(6):2043–2052. [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Rice N. S. Trabeculectomy combined with beta irradiation for congenital glaucoma. Br J Ophthalmol. 1991 Oct;75(10):584–590. doi: 10.1136/bjo.75.10.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevárez J. A., Parrish R. K., 2nd, Heuer D. K., Hajek A. S., Houdek P. V., Mallick K. S. The effect of beta irradiation on monkey Tenon's capsule fibroblasts in tissue culture. Curr Eye Res. 1987 May;6(5):719–723. doi: 10.3109/02713688709034835. [DOI] [PubMed] [Google Scholar]
- Newhouse R. P., Beyrer C. Hypotony as a late complication of trabeculectomy. Ann Ophthalmol. 1982 Jul;14(7):685–686. [PubMed] [Google Scholar]
- Occleston N. L., Alexander R. A., Mazure A., Larkin G., Khaw P. T. Effects of single exposures to antiproliferative agents on ocular fibroblast-mediated collagen contraction. Invest Ophthalmol Vis Sci. 1994 Sep;35(10):3681–3690. [PubMed] [Google Scholar]
- Skuta G. L., Beeson C. C., Higginbotham E. J., Lichter P. R., Musch D. C., Bergstrom T. J., Klein T. B., Falck F. Y., Jr Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology. 1992 Mar;99(3):438–444. doi: 10.1016/s0161-6420(92)31951-7. [DOI] [PubMed] [Google Scholar]
- Stewart N., Hicks G. G., Paraskevas F., Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995 Jan 5;10(1):109–115. [PubMed] [Google Scholar]
- Tauchi H., Sawada S. Analysis of mitotic cell death caused by radiation in mouse leukaemia L5178Y cells: apoptosis is the ultimate form of cell death following mitotic failure. Int J Radiat Biol. 1994 Apr;65(4):449–455. doi: 10.1080/09553009414550521. [DOI] [PubMed] [Google Scholar]
- Tobal K., Warren W., Cooper C. S., McCartney A., Hungerford J., Lightman S. Increased expression and mutation of p53 in choroidal melanoma. Br J Cancer. 1992 Nov;66(5):900–904. doi: 10.1038/bjc.1992.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]