Abstract
AIMS—The vitreous levels of soluble intercellular adhesion molecule 1 (sICAM-1) were investigated in uveitic eyes undergoing vitrectomy for retinal detachment (RD) or other complications, and the presence of this molecule was related to disease activity and vitreous levels of the cytokine tumour necrosis factor α (TNFα), known to upregulate ICAM-1 expression on various cells. METHODS—Vitreous and serum samples from 23 patients with either active or quiescent uveitis undergoing retinal surgery were examined for the levels of immunoreactive sICAM-1 and TNFα by ELISA methods, and for the presence of biologically active TNFα. Vitreous from non-uveitic eyes with rhegmatogenous retinal detachment (RRD), macular holes or cadaveric eyes were used as controls. RESULTS—As a whole, vitreous from uveitic eyes complicated or uncomplicated by RRD contained significantly higher levels of sICAM-1 than vitreous from non-uveitic eyes with RRD alone (p < 0.0005), eyes with macular holes (p< 0.0001), or normal cadaveric vitreous (p < 0.0001). The proportion of vitreous containing >20 ng/ml sICAM-1 (> four times the normal values) was significantly higher in eyes with uveitis complicated by RRD than in those eyes without RRD (Fisher's test, p= 0.02), and although levels of sICAM-1 were higher in eyes with active uveitis than in those with quiet disease (p < 0.02), this could not be dissociated from the increase caused by RRD. There was a relation between the vitreous levels of sICAM-1 and those of immunoreactive TNFα (Spearman's correlation coefficient; r = 0.601, p = 0.006), but not between the vitreous levels of sICAM-1 and those of biologically active TNFα. CONCLUSION—Increased vitreous sICAM-1 levels and the association of this molecule with the presence of immunoreactive TNFα in uveitic eyes confirm the operation of cytokine mediated vascular reactions at the blood-retinal barrier during the development of this condition. The persistence of high vitreous levels of sICAM-1 in eyes with uveitis complicated by RRD despite previous immunosuppression may indicate a low rate of clearance of inflammatory molecules from the vitreous cavity and an exacerbation of the existing inflammatory process by the retinal detachment itself. Keywords: vitreous; retinal detachment; ICAM-1; TNFα; uveitis
Full Text
The Full Text of this article is available as a PDF (146.6 KB).
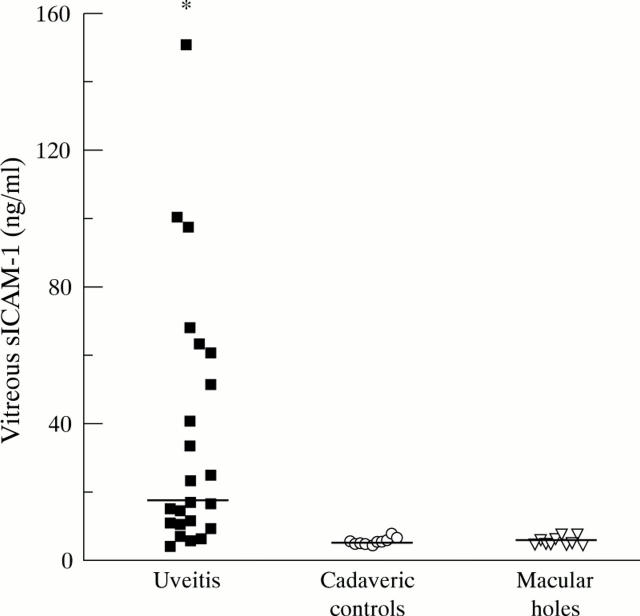
Figure 1 .
Levels of sICAM-1 in vitreous from eyes with uveitis complicated or uncomplicated by RD are significantly higher than in control cadaveric vitreous (Mann-Whitney U test, *p<0.0001) and vitreous from eyes with macular holes (Mann-Whitney U test, *p<0.0001). The bars represent median values.
Figure 2 .
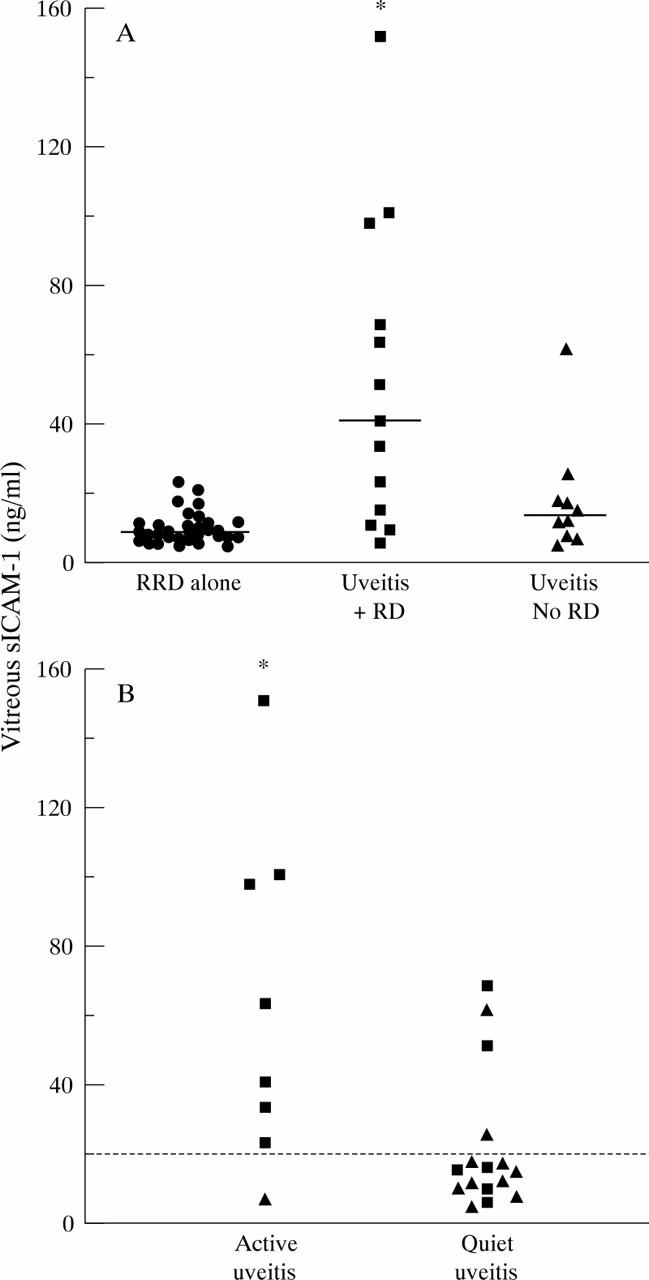
(A) Levels of sICAM-1 in vitreous from patients with uveitis complicated by retinal detachment (▪); patients with other uveitis complications but without retinal detachment (▴ ) and patients with rhegmatogenous retinal detachment alone (• ). (Mann-Whitney U test: uveitis with RD v uveitis without RD *p < 0.02; uveitis with RD v RRD alone *p < 0.0001). The bars represent median values. (B) Levels of sICAM-1 in vitreous from eyes with active uveitis compared with vitreous from eyes with quiet disease. (Mann-Whitney U test *p < 0.02). Uveitis patients with RD (▪) and without RD (▴). The dotted line represents 20 ng/ml sICAM-1.
Figure 3 .
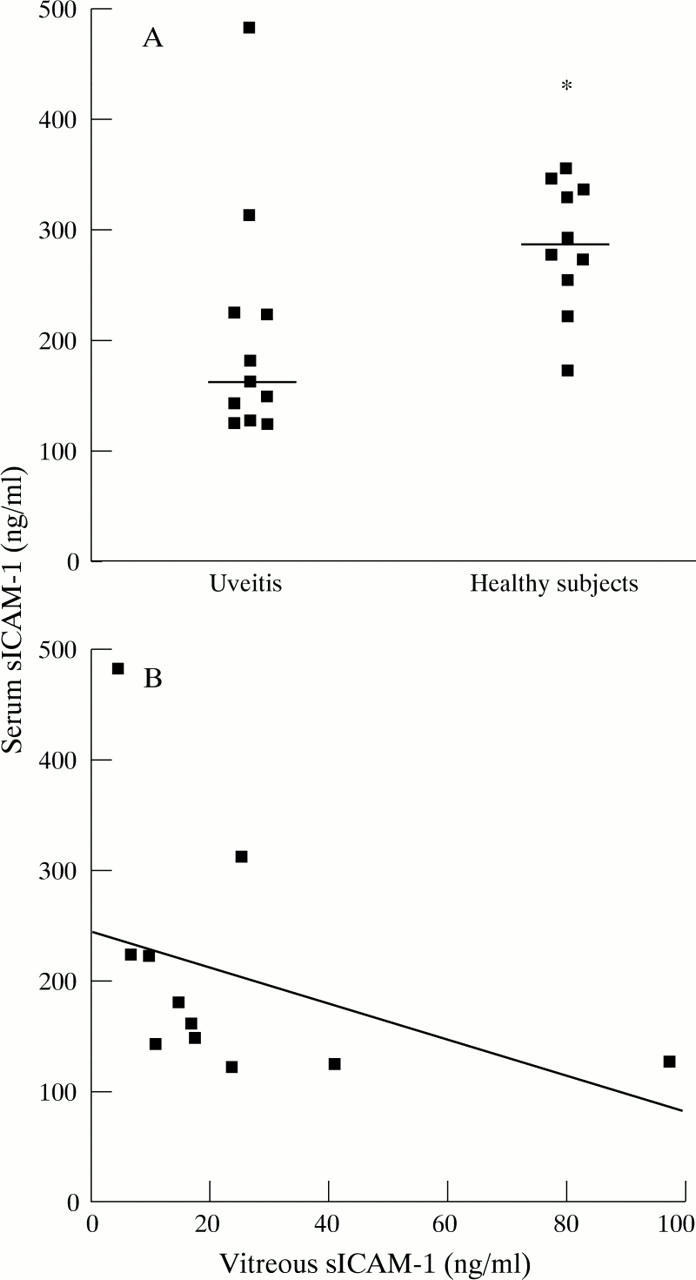
(A) Serum levels of sICAM-1 in patients with uveitis (at the time of vitrectomy) compared with those of sex and age matched controls. (Mann-Whitney U test, *p< 0.02). The bars represent median values. (B) Relation between sICAM-1 levels in serum and vitreous from uveitis patients (Mann-Whitney U test, p<0.25, r = −0.39). The solid line represents the linear regression curve of best fit.
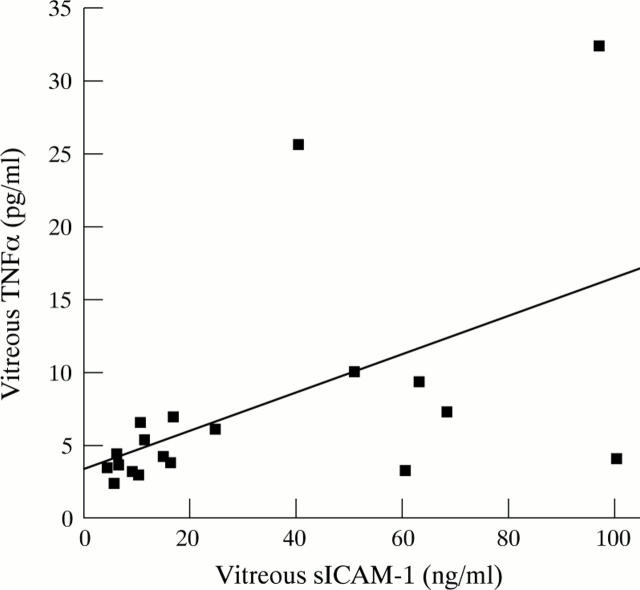
Figure 4 .
Relation between levels of sICAM-1 and immunologically detectable TNFα in vitreous from 19 patients with uveitis. The solid line represents the linear regression curve of best fit.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991 Nov 15;202(1):3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Chofflon M., Juillard C., Juillard P., Gauthier G., Grau G. E. Tumor necrosis factor alpha production as a possible predictor of relapse in patients with multiple sclerosis. Eur Cytokine Netw. 1992 Nov-Dec;3(6):523–531. [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- De Vos A. F., Van Haren M. A., Verhagen C., Hoekzema R., Kijlstra A. Tumour necrosis factor-induced uveitis in the Lewis rat is associated with intraocular interleukin 6 production. Exp Eye Res. 1995 Feb;60(2):199–207. doi: 10.1016/s0014-4835(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Pavilack M. A., Todd R. F., 3rd, Mayo-Bond L., Franklin W. A., Strieter R. M., Kunkel S. L., Huber A. R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992 Feb;66(2):200–211. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Ettehadi P., Greaves M. W., Wallach D., Aderka D., Camp R. D. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994 Apr;96(1):146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks W. A., Limb G. A., Stanford M. R., Ogilvie J., Wolstencroft R. A., Chignell A. H., Dumonde D. C. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11 (Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993 Oct;14(10):506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Kijlstra A. The role of cytokines in ocular inflammation. Br J Ophthalmol. 1994 Dec;78(12):885–886. doi: 10.1136/bjo.78.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P. S., Srinivasin B. D. Cachectin: a novel polypeptide induces uveitis in the rabbit eye. Exp Eye Res. 1988 Apr;46(4):631–633. doi: 10.1016/s0014-4835(88)80019-8. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Chignell A. H., Cole C. J., Green W. T., Webster L., Hollifield R. D., Dumonde D. C. Intercellular adhesion molecule-1 in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1997 Apr;38(5):1043–1048. [PubMed] [Google Scholar]
- Mackay C. R., Imhof B. A. Cell adhesion in the immune system. Immunol Today. 1993 Mar;14(3):99–102. doi: 10.1016/0167-5699(93)90205-Y. [DOI] [PubMed] [Google Scholar]
- Palmer H. E., Zaman A. G., Ellis B. A., Stanford M. R., Graham E. M., Wallace G. R. Longitudinal analysis of soluble intercellular adhesion molecule 1 in retinal vasculitis patients. Eur J Clin Invest. 1996 Aug;26(8):686–691. doi: 10.1111/j.1365-2362.1996.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Huang X. N., Robertson J. E., Rosenbaum J. T. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):924–930. [PubMed] [Google Scholar]
- Rocha G., Baines M. G., Deschênes J. The immunology of the eye and its systemic interactions. Crit Rev Immunol. 1992;12(3-4):81–100. [PubMed] [Google Scholar]
- Wakefield D., Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992 Jan;4(1):1–5. doi: 10.1016/1043-4666(92)90028-p. [DOI] [PubMed] [Google Scholar]
- Whitcup S. M., Chan C. C., Li Q., Nussenblatt R. B. Expression of cell adhesion molecules in posterior uveitis. Arch Ophthalmol. 1992 May;110(5):662–666. doi: 10.1001/archopht.1992.01080170084029. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Yoshimura N., Hangai M., Tanihara H., Honda Y. Interleukin-1 alpha, interleukin-1 beta, and tumor necrosis factor gene expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):1107–1113. [PubMed] [Google Scholar]
- Zaman A. G., Edelsten C., Stanford M. R., Graham E. M., Ellis B. A., Direskeneli H., D'Cruz D. P., Hughes G. R., Dumonde D. C., Wallace G. R. Soluble intercellular adhesion molecule-1 (sICAM-1) as a marker of disease relapse in idiopathic uveoretinitis. Clin Exp Immunol. 1994 Jan;95(1):60–65. doi: 10.1111/j.1365-2249.1994.tb06015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. F., Klaren V. N., Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994 Oct;35(11):3873–3883. [PubMed] [Google Scholar]