Abstract
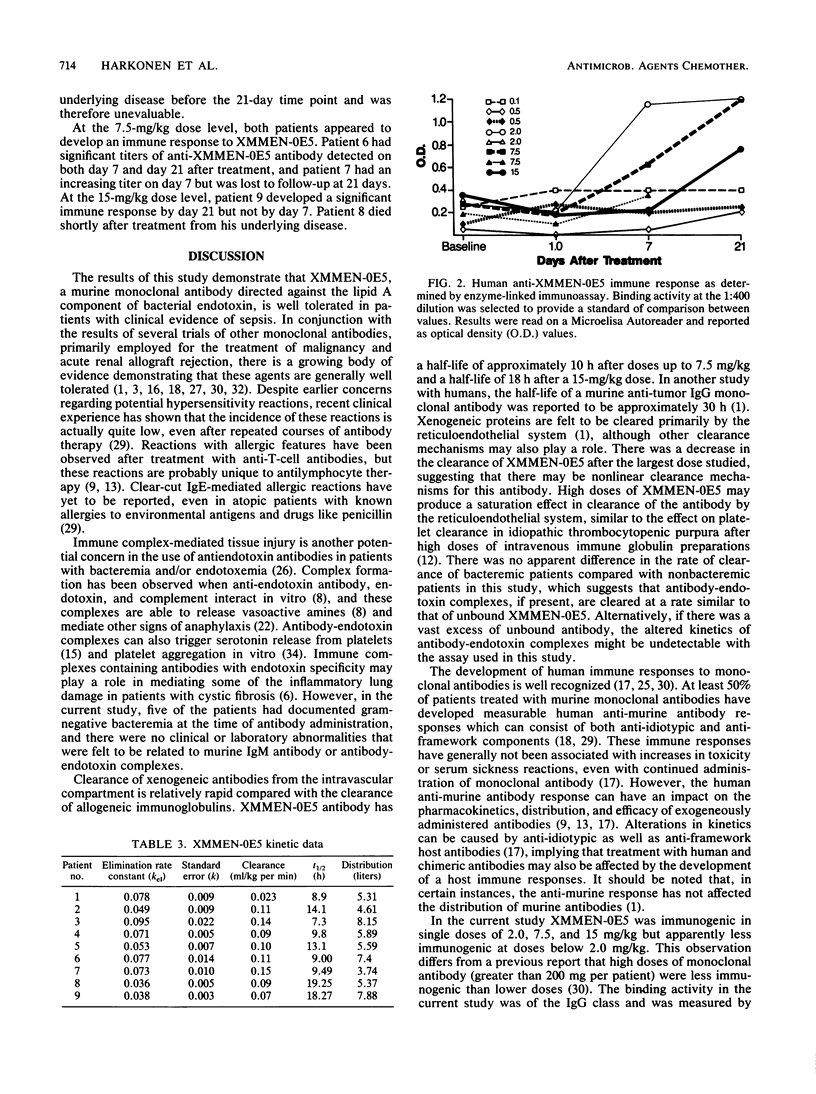
Nine patients with suspected gram-negative bacterial sepsis were studied to determine the safety, pharmacokinetics, and immunogenicity of XMMEN-0E5, a murine immunoglobulin M monoclonal antibody directed against the core lipid A region of bacterial endotoxin. Antibody was administered by single intravenous infusion of 1 to 4 h duration at doses ranging from 0.1 to 15 mg/kg. Five patients had positive blood cultures for gram-negative bacteria, one patient had Torulopsis septicemia, one patient had gram-negative bacterial meningitis, and two patients were culture negative. No evidence of antibody-mediated toxicity was observed at any dose level. The serum half-life of the antibody was approximately 10 h at doses of 0.1 to 7.5 mg/kg and approximately 18 h at a dose of 15 mg/kg. No apparent difference in clearance of antibody was observed between bacteremic and nonbacteremic patients. Human anti-mouse antibodies were detected in the sera of three evaluable patients that received doses equal to or greater than 2.0 mg/kg but not in patients that received lower doses of antibody. This study demonstrates that XMMEN-0E5 is well tolerated at doses from 0.1 to 15 mg/kg and may be immunogenic at doses of 2.0 mg/kg and above. Controlled trials to establish the efficacy of this antibody in the treatment of gram-negative bacteremia are indicated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Rapson N. T., Verweij-van Vught A. M., Maaskant J. J., Hekker T. A., Peerbooms P. G., MacLaren D. M., Thijs L. G. Heterogeneity of Escherichia coli J5 vaccines. Lancet. 1986 Nov 29;2(8518):1273–1274. doi: 10.1016/s0140-6736(86)92695-4. [DOI] [PubMed] [Google Scholar]
- Ball E. D., Bernier G. M., Cornwell G. G., 3rd, McIntyre O. R., O'Donnell J. F., Fanger M. W. Monoclonal antibodies to myeloid differentiation antigens: in vivo studies of three patients with acute myelogenous leukemia. Blood. 1983 Dec;62(6):1203–1210. [PubMed] [Google Scholar]
- Baumgartner J. D., Glauser M. P., McCutchan J. A., Ziegler E. J., van Melle G., Klauber M. R., Vogt M., Muehlen E., Luethy R., Chiolero R. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985 Jul 13;2(8446):59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Berdischewsky M., Pollack M., Young L. S., Chia D., Osher A. B., Barnett E. V. Circulating immune complexes in cystic fibrosis. Pediatr Res. 1980 Jun;14(6):830–833. doi: 10.1203/00006450-198006000-00011. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Brown K. R., Douglas H., Braude A. I. Prevention of death from endotoxin with antisera. II. Elimination of the risk of anaphylaxis to endotoxin. J Immunol. 1971 Feb;106(2):324–333. [PubMed] [Google Scholar]
- Dillman R. O., Shawler D. L., Dillman J. B., Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol. 1984 Aug;2(8):881–891. doi: 10.1200/JCO.1984.2.8.881. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Ewald D. C., Chandan N., Cerra F. B. Immunotherapy of gram-negative bacterial sepsis. A single murine monoclonal antibody provides cross-genera protection. Arch Surg. 1986 Jan;121(1):58–62. doi: 10.1001/archsurg.1986.01400010064008. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Ferguson R. M. Immunotherapy of gram-negative bacterial sepsis: enhanced survival in a guinea pig model by use of rabbit antiserum to Escherichia coli J5. Surgery. 1982 Aug;92(2):212–219. [PubMed] [Google Scholar]
- Fehr J., Hofmann V., Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med. 1982 May 27;306(21):1254–1258. doi: 10.1056/NEJM198205273062102. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Schroff R. W., Bunn P. A., Mayer D., Abrams P. G., Fer M., Ochs J., Bottino G. C., Sherwin S. A., Carlo D. J. Effects of monoclonal antibody therapy in patients with chronic lymphocytic leukemia. Blood. 1984 Nov;64(5):1085–1093. [PubMed] [Google Scholar]
- Ginsberg M. H., Henson P. M. Enhancement of platelet response to immune complexes and IgG aggregates by lipid A-rich bacterial lipopolysaccharides. J Exp Med. 1978 Jan 1;147(1):207–217. doi: 10.1084/jem.147.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N., Mintzer D., Cordon-Cardo C., Welt S., Fliegel B., Vadhan S., Carswell E., Melamed M. R., Oettgen H. F., Old L. J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman R. L., Araujo J. L., Busch G. J., Carpenter C. B., Milford E. L., Reinherz E. L., Schlossman S. F., Strom T. B., Tilney N. L. Treatment of acute renal allograft rejection with monoclonal anti-T12 antibody. Transplantation. 1983 Dec;36(6):620–626. doi: 10.1097/00007890-198336060-00005. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., McCabe W. R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980 Mar;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki D. G. Nosocomial bacteremia. An epidemiologic overview. Am J Med. 1981 Mar;70(3):719–732. doi: 10.1016/0002-9343(81)90603-3. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- McLean A. J. Anaphylactic reactions to endotoxin in guinea-pig tissues: relationship to endotoxin toxicity. Br J Pharmacol. 1977 Jul;60(3):369–373. doi: 10.1111/j.1476-5381.1977.tb07510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., McKillop J., Levy R. In vivo effects of murine hybridoma monoclonal antibody in a patient with T-cell leukemia. Blood. 1981 Jul;58(1):78–86. [PubMed] [Google Scholar]
- Ritz J., Schlossman S. F. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982 Jan;59(1):1–11. [PubMed] [Google Scholar]
- Shenep J. L., Mogan K. A. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984 Sep;150(3):380–388. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- Walker R. I., Beasley W. J. Evidence that antibodies to polysaccharide alter platelet responses to endotoxin in tolerant rabbits. Can J Microbiol. 1980 Oct;26(10):1241–1246. doi: 10.1139/m80-206. [DOI] [PubMed] [Google Scholar]
- Wolff S. M. Biological effects of bacterial endotoxins in man. J Infect Dis. 1973 Jul;128(Suppl):259–264. doi: 10.1093/infdis/128.supplement_1.s259. [DOI] [PubMed] [Google Scholar]
- Wolff S. M. The treatment of gram-negative bacteremia and shock. N Engl J Med. 1982 Nov 11;307(20):1267–1268. doi: 10.1056/NEJM198211113072010. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


