Abstract
AIMS—To investigate the adhesion characteristics of several intraocular lenses (IOLs) to the simulated and rabbit lens capsule. METHODS—Adhesive force to bovine collagen sheets was measured in water with polymethylmethacrylate (PMMA), three piece silicone, and acrylic foldable IOLs. In rabbit eyes, phacoemulsification and IOL implantation were performed. Three weeks later, adhesion between the anterior/posterior capsules and IOL optic was tested, and the capsule was examined histologically. RESULTS—The mean adhesive force to the collagen sheet was 1697 (SD 286) mg for acrylic foldable, 583 (49) mg for PMMA, and 0 mg for silicone IOLs (p=0.0003, Kruskal-Wallis test). Scores (0-5) of adhesion between rabbit anterior capsule and IOL optic were 4.50 (0.55) for acrylic foldable, 3.20 (0.84) for PMMA, and 0.40 (0.55) for silicone IOLs (p=0.004). Scores between rabbit posterior capsule and IOL optic displayed a similar tendency; 4.50 (0.84) for acrylic foldable, 3.00 (1.00) for PMMA, and 0.40 (0.55) for silicone IOLs (p=0.021). Histological observation indicated that the edge of IOL optic suppressed the migration of lens epithelial cells towards the centre of the posterior capsule. This inhibitory effect was most pronounced with acrylic foldable IOL and least with silicone IOL. CONCLUSIONS—The acrylic foldable IOL adhered to the lens capsule more than the PMMA IOL, and the silicone IOL showed no adhesiveness. These differences seem to play a role in preventing lens epithelial cells from migrating and forming posterior capsule opacification. Keywords: intraocular lens; lens capsule; posterior capsule opacification; adhesion
Full Text
The Full Text of this article is available as a PDF (148.9 KB).
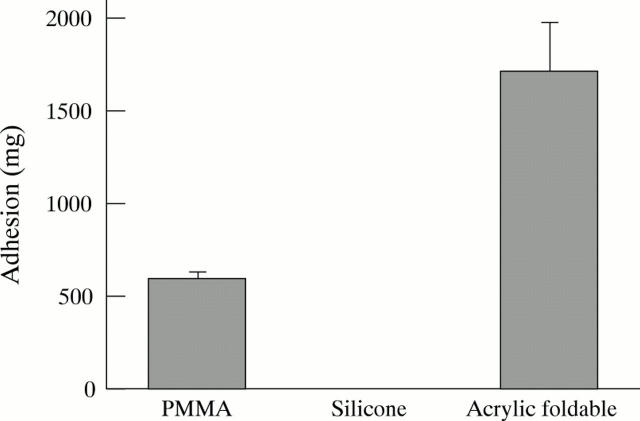
Figure 1 .
Adhesive force to collagen sheets (mean (SD)). Values were significantly different among the three groups (p=0.0003, Kruskal-Wallis test). Intergroup difference was also statistically significant between each group (p<0.001, Mann-Whitney U test).
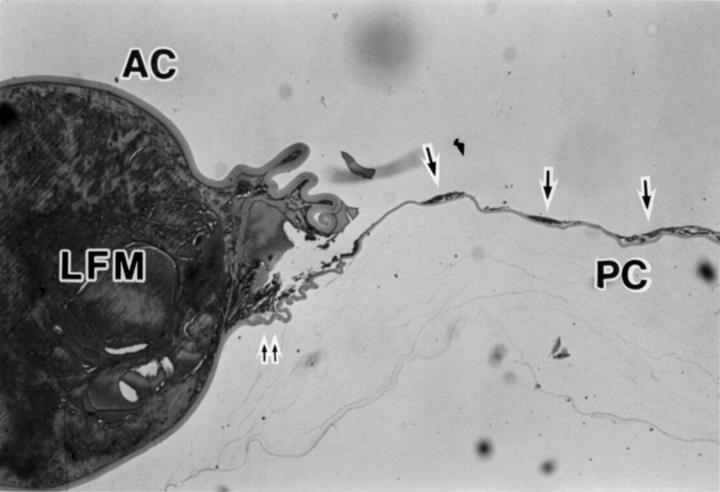
Figure 2 .
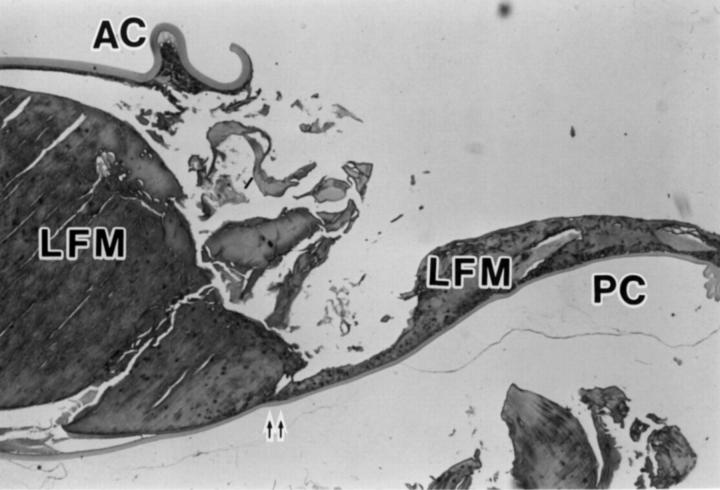
Photomicrograph of a sagittal cross section of lens capsule after removal of PMMA IOL illustrating anterior capsule (AC), posterior capsule (PC), lens fibre material (LFM), lens epithelial cells (arrows), and the position corresponding to the site where the edge of IOL optic was present (double arrow) (haematoxylin and eosin, × 12.5).
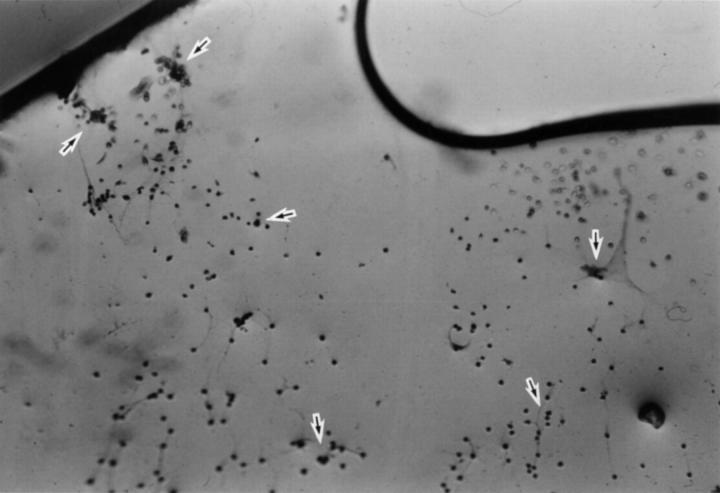
Figure 3 .
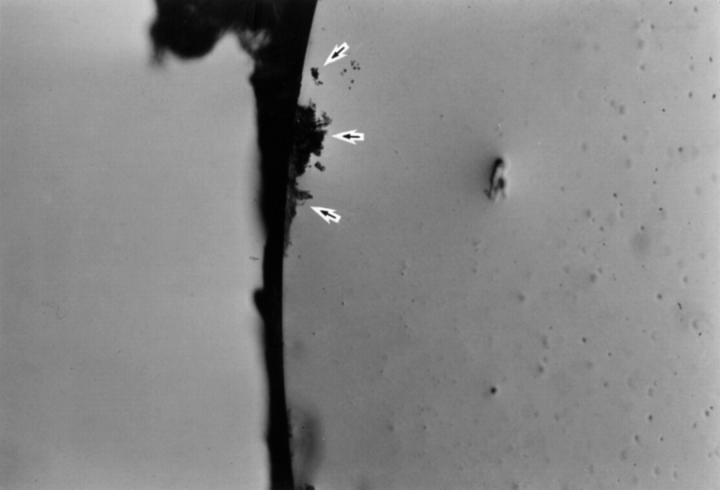
Posterior surface of PMMA IOL after removal from the capsular bag. Lens epithelial cells are scattered across the surface (arrows).
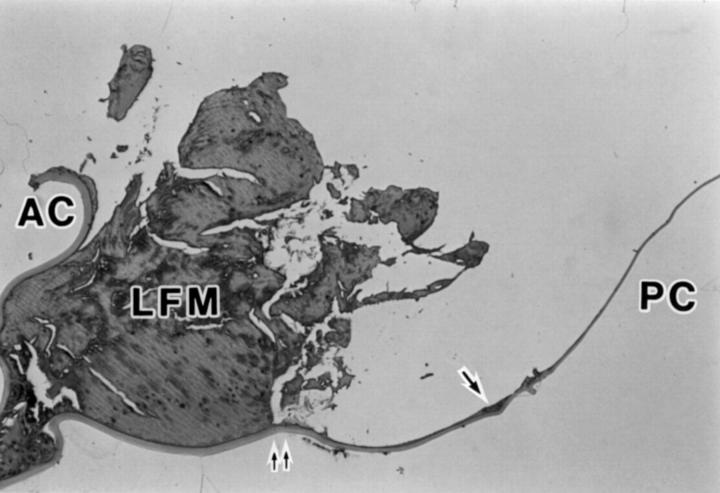
Figure 4 .
Photomicrograph of a sagittal cross section of lens capsule after removal of acrylic foldable IOL illustrating anterior capsule (AC), posterior capsule (PC), lens fibre material (LFM), lens epithelial cells (arrows), and the position corresponding to the site where the edge of IOL optic was present (double arrow) (haematoxylin and eosin, × 12.5).
Figure 5 .
Posterior surface of acrylic foldable IOL after removal from the capsular bag. Lens epithelial cells are clustered at the edge (arrows).
Figure 6 .
Photomicrograph of a sagittal cross section of lens capsule after removal of silicone IOL illustrating anterior capsule (AC), posterior capsule (PC), lens fibre material (LFM), and the position corresponding to the site where the edge of IOL optic was present (double arrow) (haematoxylin and eosin, × 12.5).
Figure 7 .
Posterior surface of silicone IOL after removal from the capsular bag.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple D. J., Solomon K. D., Tetz M. R., Assia E. I., Holland E. Y., Legler U. F., Tsai J. C., Castaneda V. E., Hoggatt J. P., Kostick A. M. Posterior capsule opacification. Surv Ophthalmol. 1992 Sep-Oct;37(2):73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Born C. P., Ryan D. K. Effect of intraocular lens optic design on posterior capsular opacification. J Cataract Refract Surg. 1990 Mar;16(2):188–192. doi: 10.1016/s0886-3350(13)80728-6. [DOI] [PubMed] [Google Scholar]
- Cammarata P. R., Cantu-Crouch D., Oakford L., Morrill A. Macromolecular organization of bovine lens capsule. Tissue Cell. 1986;18(1):83–97. doi: 10.1016/0040-8166(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Duncker G. I., Westphalen S., Behrendt S. Complications of silicone disc intraocular lenses. J Cataract Refract Surg. 1995 Sep;21(5):562–566. doi: 10.1016/s0886-3350(13)80218-0. [DOI] [PubMed] [Google Scholar]
- Frezzotti R., Caporossi A. Pathogenesis of posterior capsular opacification. Part I. Epidemiological and clinico-statistical data. J Cataract Refract Surg. 1990 May;16(3):347–352. doi: 10.1016/s0886-3350(13)80707-9. [DOI] [PubMed] [Google Scholar]
- Gwon A., Gruber L. J., Mantras C. Restoring lens capsule integrity enhances lens regeneration in New Zealand albino rabbits and cats. J Cataract Refract Surg. 1993 Nov;19(6):735–746. doi: 10.1016/s0886-3350(13)80343-4. [DOI] [PubMed] [Google Scholar]
- Hansen T. E., Otland N., Corydon L. Posterior capsule fibrosis and intraocular lens design. J Cataract Refract Surg. 1988 Jul;14(4):383–386. doi: 10.1016/s0886-3350(88)80143-3. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara T., Sakanishi K., Yamada Y. Efficacy of equator rings in an experimental rabbit study. Arch Ophthalmol. 1995 Aug;113(8):1060–1065. doi: 10.1001/archopht.1995.01100080112038. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi H., Nakao F., Hayashi F. Capsular capture of silicone intraocular lenses. J Cataract Refract Surg. 1996;22 (Suppl 2):1267–1271. doi: 10.1016/s0886-3350(96)80082-4. [DOI] [PubMed] [Google Scholar]
- Kappelhof J. P., Vrensen G. F. The pathology of after-cataract. A minireview. Acta Ophthalmol Suppl. 1992;(205):13–24. doi: 10.1111/j.1755-3768.1992.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Kennedy A., Frank R. N., Mancini M. A. In vitro production of glycosaminoglycans by retinal microvessel cells and lens epithelium. Invest Ophthalmol Vis Sci. 1986 May;27(5):746–754. [PubMed] [Google Scholar]
- Koo E. Y., Lindsey P. S., Soukiasian S. H. Bisecting a foldable acrylic intraocular lens for explantation. J Cataract Refract Surg. 1996;22 (Suppl 2):1381–1382. doi: 10.1016/s0886-3350(96)80103-9. [DOI] [PubMed] [Google Scholar]
- Lindstrom R. L., Harris W. S. Management of the posterior capsule following posterior chamber lens implantation. J Am Intraocul Implant Soc. 1980 Jul;6(3):255–258. doi: 10.1016/s0146-2776(80)80073-5. [DOI] [PubMed] [Google Scholar]
- Mamalis N., Phillips B., Kopp C. H., Crandall A. S., Olson R. J. Neodymium: YAG capsulotomy rates after phacoemulsification with silicone posterior chamber intraocular lenses. J Cataract Refract Surg. 1996;22 (Suppl 2):1296–1302. doi: 10.1016/s0886-3350(96)80088-5. [DOI] [PubMed] [Google Scholar]
- Milauskas A. T. Posterior capsule opacification after silicone lens implantation and its management. J Cataract Refract Surg. 1987 Nov;13(6):644–648. doi: 10.1016/s0886-3350(87)80155-4. [DOI] [PubMed] [Google Scholar]
- Nagamoto T., Hara E. Lens epithelial cell migration onto the posterior capsule in vitro. J Cataract Refract Surg. 1996;22 (Suppl 1):841–846. doi: 10.1016/s0886-3350(96)80172-6. [DOI] [PubMed] [Google Scholar]
- Nasisse M. P., Dykstra M. J., Cobo L. M. Lens capsule opacification in aphakic and pseudophakic eyes. Graefes Arch Clin Exp Ophthalmol. 1995 Feb;233(2):63–70. doi: 10.1007/BF00241473. [DOI] [PubMed] [Google Scholar]
- Neuhann T. H. Intraocular folding of an acrylic lens for explantation through a small incision cataract wound. J Cataract Refract Surg. 1996;22 (Suppl 2):1383–1386. doi: 10.1016/s0886-3350(96)80104-0. [DOI] [PubMed] [Google Scholar]
- Nishi O. Incidence of posterior capsule opacification in eyes with and without posterior chamber intraocular lenses. J Cataract Refract Surg. 1986 Sep;12(5):519–522. doi: 10.1016/s0886-3350(86)80126-2. [DOI] [PubMed] [Google Scholar]
- Oshika T., Suzuki Y., Kizaki H., Yaguchi S. Two year clinical study of a soft acrylic intraocular lens. J Cataract Refract Surg. 1996 Jan-Feb;22(1):104–109. doi: 10.1016/s0886-3350(96)80278-1. [DOI] [PubMed] [Google Scholar]
- Parmigiani C. M., McAvoy J. W. The roles of laminin and fibronectin in the development of the lens capsule. Curr Eye Res. 1991 Jun;10(6):501–511. doi: 10.3109/02713689109001758. [DOI] [PubMed] [Google Scholar]
- Sanchez E., Artaria L. Evaluation of the first 50 ACR360 acrylic intraocular lens implantations. J Cataract Refract Surg. 1996;22 (Suppl 2):1373–1378. doi: 10.1016/s0886-3350(96)80101-5. [DOI] [PubMed] [Google Scholar]
- Santos B. A., Pastora R., DelMonte M. A., O'Donnell F. E., Jr Comparative study of the effects of optic design on lens epithelium in vitro. J Cataract Refract Surg. 1987 Mar;13(2):127–130. doi: 10.1016/s0886-3350(87)80125-6. [DOI] [PubMed] [Google Scholar]
- Sellman T. R., Lindstrom R. L. Effect of a plano-convex posterior chamber lens on capsular opacification from Elschnig pearl formation. J Cataract Refract Surg. 1988 Jan;14(1):68–72. doi: 10.1016/s0886-3350(88)80067-1. [DOI] [PubMed] [Google Scholar]
- Sterling S., Wood T. O. Effect of intraocular lens convexity on posterior capsule opacification. J Cataract Refract Surg. 1986 Nov;12(6):655–657. doi: 10.1016/s0886-3350(86)80080-3. [DOI] [PubMed] [Google Scholar]
- Yamada K., Nagamoto T., Yozawa H., Kato K., Kurosaka D., Miyajima H. B., Kimura C. Effect of intraocular lens design on posterior capsule opacification after continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1995 Nov;21(6):697–700. doi: 10.1016/s0886-3350(13)80569-x. [DOI] [PubMed] [Google Scholar]
- Zetterström C., Kugelberg U., Lundgren B., Syrén-Nordqvist S. After-cataract formation in newborn rabbits implanted with intraocular lenses. J Cataract Refract Surg. 1996 Jan-Feb;22(1):85–88. doi: 10.1016/s0886-3350(96)80275-6. [DOI] [PubMed] [Google Scholar]