Abstract
AIM—To investigate the effects of single, short term (5 or 30 minutes) exposures to thiotepa or 5-fluorouracil (5-FU) on collagen lattice contraction and retinal pigment epithelial (RPE) cell proliferation. METHODS—For collagen contraction studies, RPE cells seeded into free floating type I collagen lattices were exposed to single 5 or 30 minute treatments with thiotepa (0.06-4 mg/ml), or 5-FU (0.25-25 mg/ml), or phosphate buffered saline alone as a control. For proliferation studies, RPE cell monolayers were similarly exposed to these agents. The degree of contraction, effects on cell number, and viability were determined up to 14 days after treatment. RESULTS—Contraction of collagen lattices containing RPE cells and proliferation of RPE cells were significantly inhibited (p<0.05) by thiotepa and 5-FU at concentrations above 0.06 mg/ml and 0.25 mg/ml respectively (for both 5 and 30 minute treatments), compared with controls. Cell death did not occur except for exposure of the RPE cells in collagen lattices to the highest concentration of thiotepa (4 mg/ml). CONCLUSION—It was concluded that single 5 or 30 minute exposures to thiotepa or 5-FU significantly inhibited collagen contraction and the proliferation of RPE cells. These findings suggest that short, single, non-toxic exposures to thiotepa or 5-FU which can be reproduced clinically may be useful in the modulation of proliferative vitreoretinopathy. Keywords: proliferative vitreoretinopathy; collagen matrix contraction; thiotepa; 5-fluorouracil
Full Text
The Full Text of this article is available as a PDF (112.7 KB).
Figure 1 .
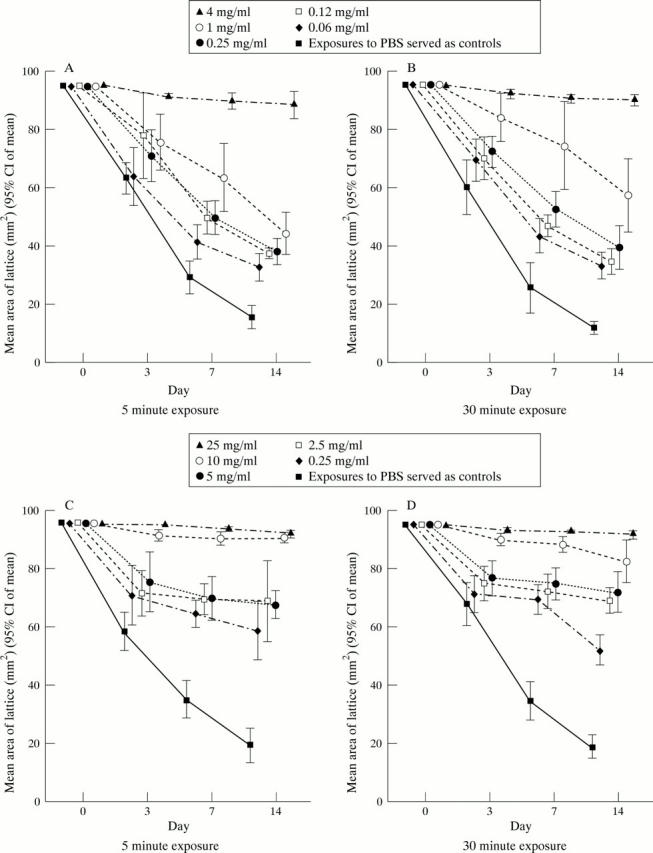
Effect of exposure to thiotepa (A and B) or 5-FU (C and D) on collagen lattice contraction.
Figure 2 .
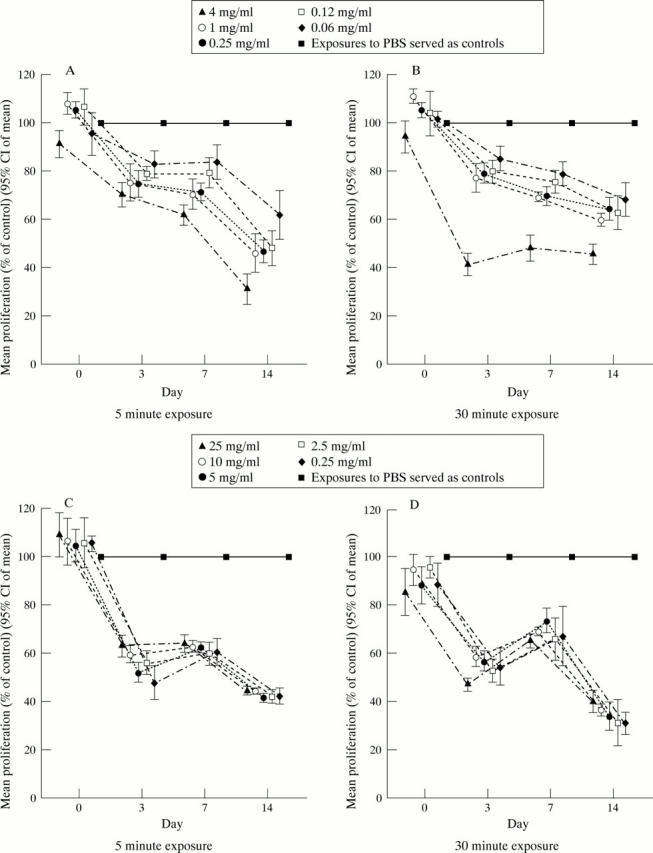
Effect of exposure to thiotepa (A and B) or 5-FU (C and D) on RPE cell proliferation (using a colorimetric test).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrada A., Peyman G. A., Case J., Fishman G., Thomas A., Fiscella R. Evaluation of intravitreal 5-fluorouracil, vincristine, VP 16, doxorubicin, and thiotepa in primate eyes. Ophthalmic Surg. 1984 Sep;15(9):767–769. [PubMed] [Google Scholar]
- Blumenkranz M. S., Hartzer M. K., Hajek A. S. Selection of therapeutic agents for intraocular proliferative disease. II. Differing antiproliferative activity of the fluoropyrimidines. Arch Ophthalmol. 1987 Mar;105(3):396–399. doi: 10.1001/archopht.1987.01060030116039. [DOI] [PubMed] [Google Scholar]
- Blumenkranz M. S., Ophir A., Claflin A. J., Hajek A. Fluorouracil for the treatment of massive periretinal proliferation. Am J Ophthalmol. 1982 Oct;94(4):458–467. doi: 10.1016/0002-9394(82)90239-2. [DOI] [PubMed] [Google Scholar]
- ERICSON L. A., ROSENGREN B. H. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569–576. doi: 10.1111/j.1755-3768.1961.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Edwards R. B. Culture of mammalian retinal pigment epithelium and neural retina. Methods Enzymol. 1982;81:39–43. doi: 10.1016/s0076-6879(82)81008-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich D. The management of pterygium. Ophthalmic Surg. 1977 Apr;8(2):23–30. [PubMed] [Google Scholar]
- Ericson L., Karlberg B., Rosengren B. H. Trials of intravitreal injections of chemotherapeutic agents in rabbits. Acta Ophthalmol (Copenh) 1964;42(4):721–726. doi: 10.1111/j.1755-3768.1964.tb01723.x. [DOI] [PubMed] [Google Scholar]
- Hartzer M. K., Blumenkranz M. S., Hajek A. S., Dailey W. A., Cheng M., Margherio A. R. Selection of therapeutic agents for intraocular proliferative disease 3. Effects of fluoropyrimidines on cell-mediated contraction of human fibroblasts. Exp Eye Res. 1989 Mar;48(3):321–328. doi: 10.1016/s0014-4835(89)80001-6. [DOI] [PubMed] [Google Scholar]
- Hiscott P. S., Grierson I., McLeod D. Retinal pigment epithelial cells in epiretinal membranes: an immunohistochemical study. Br J Ophthalmol. 1984 Oct;68(10):708–715. doi: 10.1136/bjo.68.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerdan J. A., Pepose J. S., Michels R. G., Hayashi H., de Bustros S., Sebag M., Glaser B. M. Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology. 1989 Jun;96(6):801–810. doi: 10.1016/s0161-6420(89)32818-1. [DOI] [PubMed] [Google Scholar]
- Karan D. M., Macey R. I. The permeability of the human red cell to deuterium oxide (heavy water). J Cell Physiol. 1980 Aug;104(2):209–214. doi: 10.1002/jcp.1041040210. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Doyle J. W., Sherwood M. B., Grierson I., Schultz G., McGorray S. Prolonged localized tissue effects from 5-minute exposures to fluorouracil and mitomycin C. Arch Ophthalmol. 1993 Feb;111(2):263–267. doi: 10.1001/archopht.1993.01090020117035. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Migdal C. S. Current techniques in wound healing modulation in glaucoma surgery. Curr Opin Ophthalmol. 1996 Apr;7(2):24–33. doi: 10.1097/00055735-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Ward S., Grierson I., Rice N. S. Effect of beta radiation on proliferating human Tenon's capsule fibroblasts. Br J Ophthalmol. 1991 Oct;75(10):580–583. doi: 10.1136/bjo.75.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw P. T., Ward S., Porter A., Grierson I., Hitchings R. A., Rice N. S. The long-term effects of 5-fluorouracil and sodium butyrate on human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 1992 May;33(6):2043–2052. [PubMed] [Google Scholar]
- Machemer R., Laqua H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol. 1975 Jul;80(1):1–23. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- Mandelcorn M. S., Machemer R., Fineberg E., Hersch S. B. Proliferation and metaplasia of intravitreal retinal pigment epithelium cell autotransplants. Am J Ophthalmol. 1975 Aug;80(2):227–237. doi: 10.1016/0002-9394(75)90137-3. [DOI] [PubMed] [Google Scholar]
- Morino I., Hiscott P., McKechnie N., Grierson I. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol. 1990 Jul;74(7):393–399. doi: 10.1136/bjo.74.7.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Occleston N. L., Alexander R. A., Mazure A., Larkin G., Khaw P. T. Effects of single exposures to antiproliferative agents on ocular fibroblast-mediated collagen contraction. Invest Ophthalmol Vis Sci. 1994 Sep;35(10):3681–3690. [PubMed] [Google Scholar]
- Scheiffarth O. F., Kampik A., Günther H., von der Mark K. Proteins of the extracellular matrix in vitreoretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):357–361. doi: 10.1007/BF02172967. [DOI] [PubMed] [Google Scholar]
- Seregard S., Kock E., af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995 Feb;79(2):194–195. doi: 10.1136/bjo.79.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]


