Abstract
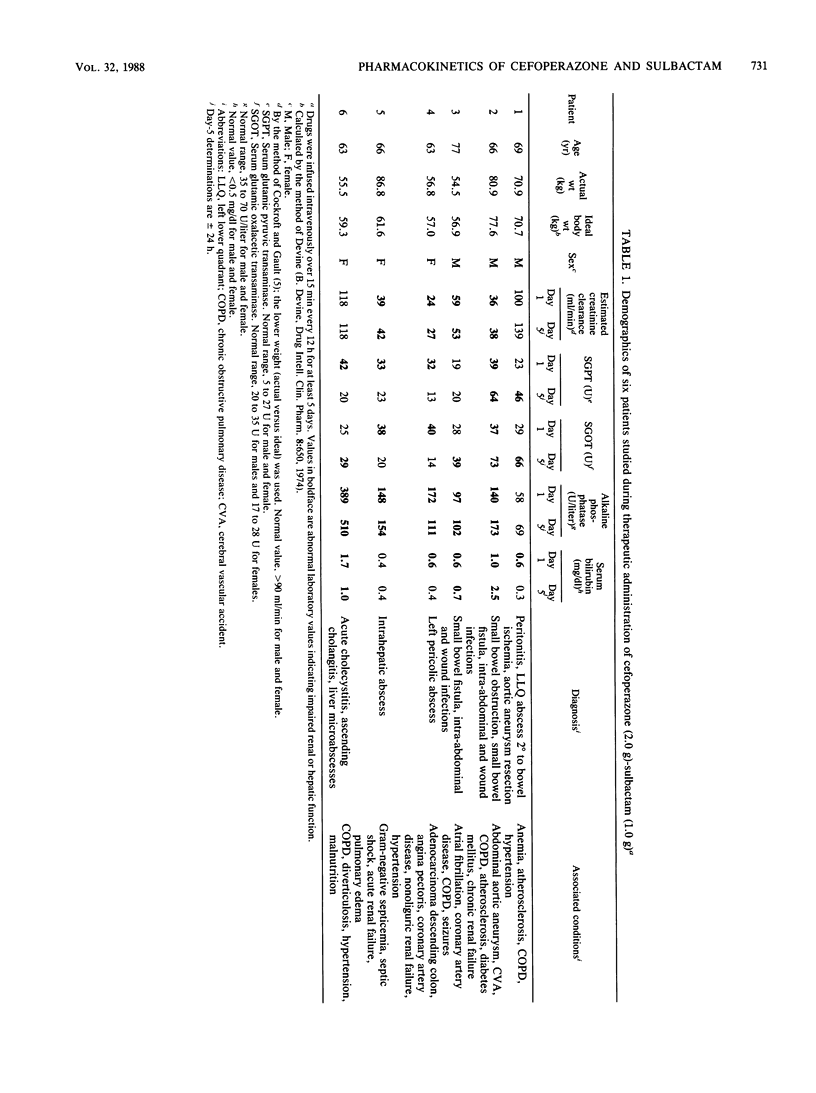
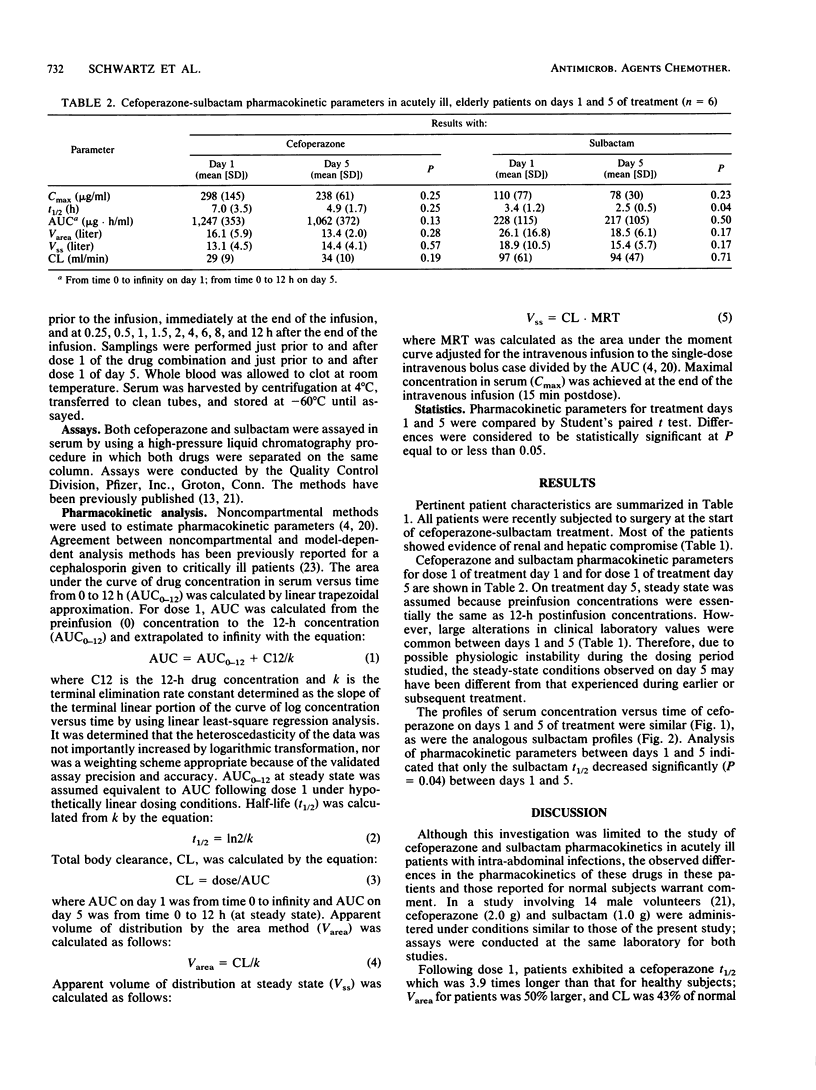
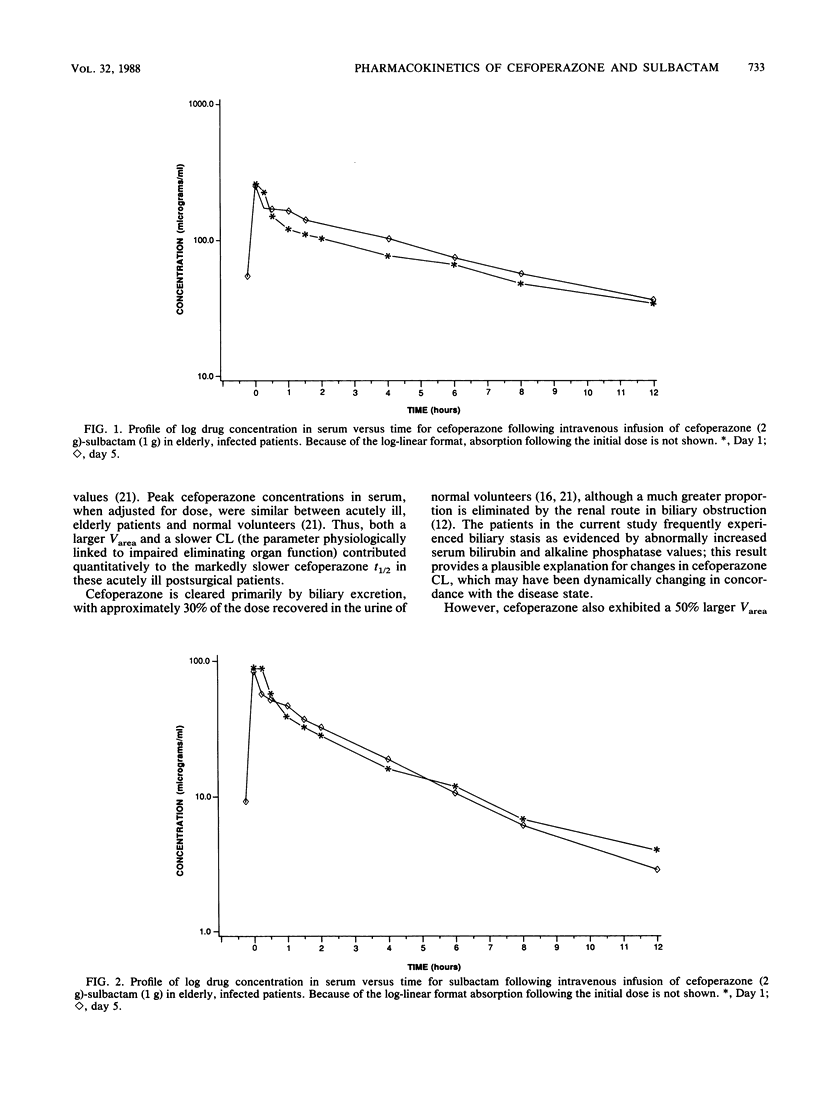
The pharmacokinetics of cefoperazone and sulbactam in combination were evaluated in six, elderly, seriously ill patients treated with the drug combination for intra-abdominal infections. After giving informed consent, three males and three females aged 63.5 to 77.5 (mean 67.9) years and weighing 54.5 to 86.8 (mean, 67.6) kg were treated with cefoperazone (2.0 g) and sulbactam (1.0 g) infused intravenously every 12 h for at least 5 days. Cefoperazone and sulbactam pharmacokinetics were characterized on both days 1 and 5 of treatment. Eleven serial blood samples were obtained just prior to and following dose 1 on days 1 and 5 of treatment. Mean estimates of cefoperazone maximal concentration in plasma (Cmax), area under the curve of drug concentration in plasma versus time (AUC), half-life (t 1/2), apparent volume of distribution by the area method (Varea), apparent volume of distribution at steady state (Vss), and total body clearance (CL) for day 1 (day 5) were 297.5 237.5) micrograms/ml, 1,247 (1,063) micrograms.h/ml, 7.0 (4.9) h, 16.1 (13.4) liter, 13.1 (14.4) liter, and 28.9 (34.2) ml/min, respectively. Day 1 (day 5) mean values for sulbactam Cmax, AUC, t 1/2, Varea, Vss, and CL were 110.3 (78.0) micrograms/ml, 228 (217) micrograms.h/ml, 3.4 (2.5) h, 26.1 (18.5) liter, 18.9 (15.4) liter, and 97 (94) ml/min, respectively. Both drugs evidenced slower elimination and greater pharmacokinetic variability in these patients compared with values previously reported for normal volunteers. As patients improved during the course of therapy, the only pharmacokinetic parameter significantly changed between days 1 and 5 was a shortened sulbactam t 1/2. Our inability to find substantial evidence of pharmacokinetic normalization may have been related to sample size and study duration. Both drugs were present in potentially therapeutic concentrations for the entire 12-h dosing interval, but without undue accumulation from days 1 to 5.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balant L., Dayer P., Rudhardt M., Allaz A. F., Fabre J. Cefoperazone: pharmacokinetics in humans with normal and impaired renal function and pharmacokinetics in rats. Clin Ther. 1980;3(Spec Issue):50–59. [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Sande M. A. Pharmacokinetics of cefoperazone in normal volunteers and subjects with renal insufficiency. Antimicrob Agents Chemother. 1981 May;19(5):821–825. doi: 10.1128/aac.19.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M., Hancox J. Comparative pharmacokinetics and tissue penetration of sulbactam and ampicillin after concurrent intravenous administration. Antimicrob Agents Chemother. 1982 Apr;21(4):565–567. doi: 10.1128/aac.21.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1984 Apr;73(4):570–571. doi: 10.1002/jps.2600730436. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Gerber A. U. Pharmacokinetics of cefoperazone: a review. Drugs. 1981;22 (Suppl 1):35–45. doi: 10.2165/00003495-198100221-00010. [DOI] [PubMed] [Google Scholar]
- Craig W. A. Single-dose pharmacokinetics of cefoperazone following intravenous administration. Clin Ther. 1980;3(Spec Issue):46–49. [PubMed] [Google Scholar]
- Craig W. A., Welling P. G. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1977 Jul-Aug;2(4):252–268. doi: 10.2165/00003088-197702040-00002. [DOI] [PubMed] [Google Scholar]
- Cunha B. A., Ristuccia A. M. Third generation cephalosporins. Med Clin North Am. 1982 Jan;66(1):283–291. doi: 10.1016/s0025-7125(16)31460-2. [DOI] [PubMed] [Google Scholar]
- Foulds G., Stankewich J. P., Marshall D. C., O'Brien M. M., Hayes S. L., Weidler D. J., McMahon F. G. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother. 1983 May;23(5):692–699. doi: 10.1128/aac.23.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M. A., Kern J. W., Chenella F. C., Heseltine P. N., Yellin A. E., Foulds G., Berne T. V. Pharmacokinetics of parenteral sulbactam in patients with appendicitis. Ther Drug Monit. 1984;6(4):428–431. doi: 10.1097/00007691-198412000-00008. [DOI] [PubMed] [Google Scholar]
- Greenfield R. A., Gerber A. U., Craig W. A. Pharmacokinetics of cefoperazone in patients with normal and impaired hepatic and renal function. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S127–S136. doi: 10.1093/clinids/5-supplement_1.s127. [DOI] [PubMed] [Google Scholar]
- Johnson C. A., Zimmerman S. W., Reitberg D. P., Whall T. J., Leggett J. E., Craig W. A. Pharmacokinetics and pharmacodynamics of cefoperazone-sulbactam in patients on continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1988 Jan;32(1):51–56. doi: 10.1128/aac.32.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labia R., Lelievre V., Peduzzi J. Inhibition kinetics of three R-factor-mediated beta-lactamases by a new beta-lactam sulfone (CP 45899). Biochim Biophys Acta. 1980 Feb 14;611(2):351–357. doi: 10.1016/0005-2744(80)90071-6. [DOI] [PubMed] [Google Scholar]
- Neu H. C. A review and summary of the pharmacokinetics of cefoperazone: a new, extended-spectrum beta-lactam antibiotic. Ther Drug Monit. 1981;3(2):121–128. doi: 10.1097/00007691-198102000-00002. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. Annu Rev Pharmacol Toxicol. 1982;22:599–642. doi: 10.1146/annurev.pa.22.040182.003123. [DOI] [PubMed] [Google Scholar]
- Reitberg D. P., Cumbo T. J., Smith I. L., Schentag J. J. Effect of protein binding on cefmenoxime steady-state kinetics in critical patients. Clin Pharmacol Ther. 1984 Jan;35(1):64–73. doi: 10.1038/clpt.1984.10. [DOI] [PubMed] [Google Scholar]
- Reitberg D. P., Marble D. A., Schultz R. W., Whall T. J., Schentag J. J. Pharmacokinetics of cefoperazone (2.0 g) and sulbactam (1.0 g) coadministered to subjects with normal renal function, patients with decreased renal function, and patients with end-stage renal disease on hemodialysis. Antimicrob Agents Chemother. 1988 Apr;32(4):503–509. doi: 10.1128/aac.32.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitberg D. P., Smith I. L., Love S. J., Lewin H. M., Schentag J. J. A rapid, universal TI-59 model-independent pharmacokinetic analysis program based on statistical moment theory. Drug Intell Clin Pharm. 1985 Feb;19(2):125–134. doi: 10.1177/106002808501900209. [DOI] [PubMed] [Google Scholar]
- Reitberg D. P., Whall T. J., Chung M., Blickens D., Swarz H., Arnold J. Multiple-dose pharmacokinetics and toleration of intravenously administered cefoperazone and sulbactam when given as single agents or in combination. Antimicrob Agents Chemother. 1988 Jan;32(1):42–46. doi: 10.1128/aac.32.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Swanson D. J., Reitberg D. P., Smith I. L., Wels P. B., Schentag J. J. Steady-state moxalactam pharmacokinetics in patients: noncompartmental versus two-compartmental analysis. J Pharmacokinet Biopharm. 1983 Aug;11(4):337–353. doi: 10.1007/BF01058954. [DOI] [PubMed] [Google Scholar]


