Abstract
AIMS—Previous studies have implied that interferon gamma (IFN-γ) is involved in the pathogenesis of endotoxin induced uveitis (EIU) in the rat. This study investigated the source of IFN-γ in the iris during EIU. METHODS—Whole mounts of iris were isolated from Lewis rats before and at different times (from 4 hours to 14 days) after foot pad injection of 200 µg Salmonella typhimurium lipopolysaccharide (LPS). Immunohistological analysis was performed using monoclonal antibodies (mAbs) specific to rat IFN-γ (DB12 and DB13). mAbs specific to monocytes, macrophages, and dendritic cells and MHC class II were used to asses the inflammatory response in the eye (ED-1, ED-2, and OX-6). An antibody specific to neurofilaments (2H3) was used to stain nerve fibres in the normal iris. RESULTS—LPS administration induced acute intraocular inflammation, characterised by a massive infiltration of monocytes/macrophages and increased numbers of MHC class II positive cells in the iris. IFN-γ immunoreactive cells were not detected in iris whole mounts of control rats. Strikingly, IFN-γ immunoreactivity was found in fibres from 4 hours until 10 days after LPS injection, with the most intense staining at 48-72 hours. Other DB12 or DB13 positive cells were not detected in the iris. The pattern of DB12 and DB13 staining in the inflamed iris was similar to the 2H3 staining of neurons in the iris of control rats. CONCLUSION—These results show that systemic LPS administration induces IFN-γ immunoreactivity in iris fibres and suggest that iris nerve fibres may be a source of IFN-γ during EIU. The IFN-γ immunoreactive material in the iris nerve fibres may be identical to neuronal IFN-γ. Keywords: endotoxin induced uveitis; cytokines; interferon gamma; rat
Full Text
The Full Text of this article is available as a PDF (207.4 KB).
Figure 1 .
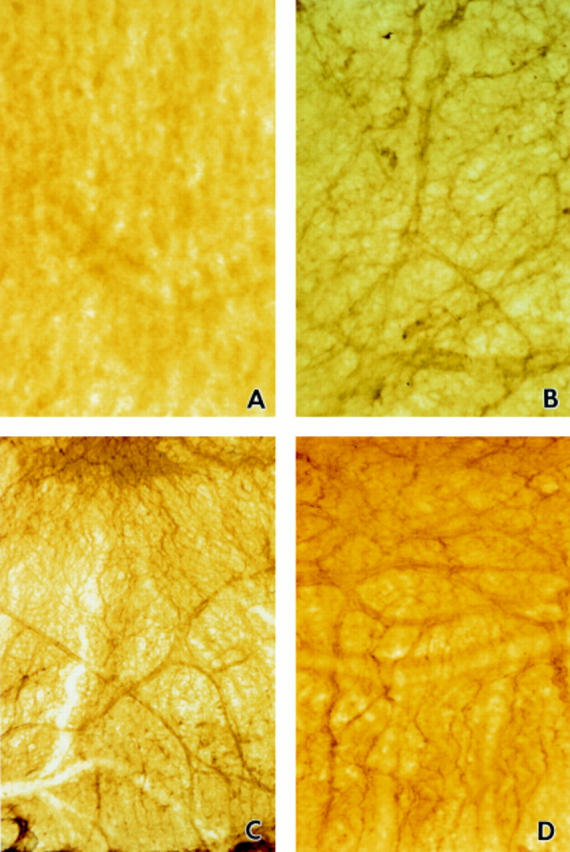
Immunohistochemical staining of iris whole mounts with mAbs DB12 (A and B) and DB13 (C and D). Iris whole mounts used were isolated from a control rat (A) and at 24 hours (B), 48 hours (C), and 72 hours (D) after LPS injection. Original magnifications, A ×250; B ×250; C ×63; and D ×100.
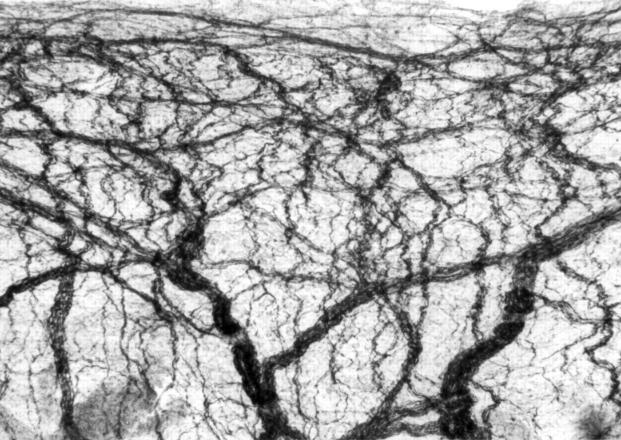
Figure 2 .
Immunohistochemical staining of an iris whole mount isolated from a control rat with mAbs 2H3. Original magnification, ×100.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scand. 1979 Jul;106(3):371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x. [DOI] [PubMed] [Google Scholar]
- De Vos A. F., Hoekzema R., Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992 Jun;11(6):581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- Dodd J., Morton S. B., Karagogeos D., Yamamoto M., Jessell T. M. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988 Apr;1(2):105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Egwuagu C. E., Sztein J., Chan C. C., Reid W., Mahdi R., Nussenblatt R. B., Chepelinsky A. B. Ectopic expression of gamma interferon in the eyes of transgenic mice induces ocular pathology and MHC class II gene expression. Invest Ophthalmol Vis Sci. 1994 Feb;35(2):332–341. [PubMed] [Google Scholar]
- Geiger K., Howes E., Gallina M., Huang X. J., Travis G. H., Sarvetnick N. Transgenic mice expressing IFN-gamma in the retina develop inflammation of the eye and photoreceptor loss. Invest Ophthalmol Vis Sci. 1994 May;35(6):2667–2681. [PubMed] [Google Scholar]
- Herbort C. P., Okumura A., Mochizuki M. Endotoxin-induced uveitis in the rat. A study of the role of inflammation mediators. Graefes Arch Clin Exp Ophthalmol. 1988;226(6):553–558. doi: 10.1007/BF02169204. [DOI] [PubMed] [Google Scholar]
- Kiefer R., Haas C. A., Kreutzberg G. W. Gamma interferon-like immunoreactive material in rat neurons: evidence against a close relationship to gamma interferon. Neuroscience. 1991;45(3):551–560. doi: 10.1016/0306-4522(91)90270-x. [DOI] [PubMed] [Google Scholar]
- Kiefer R., Kreutzberg G. W. Gamma interferon-like immunoreactivity in the rat nervous system. Neuroscience. 1990;37(3):725–734. doi: 10.1016/0306-4522(90)90103-b. [DOI] [PubMed] [Google Scholar]
- Kogiso M., Tanouchi Y., Mimura Y., Nagasawa H., Himeno K. Endotoxin-induced uveitis in mice. 1. Induction of uveitis and role of T lymphocytes. Jpn J Ophthalmol. 1992;36(3):281–290. [PubMed] [Google Scholar]
- Ljungdahl A., Olsson T., Van der Meide P. H., Holmdahl R., Klareskog L., Höjeberg B. Interferon-gamma-like immunoreactivity in certain neurons of the central and peripheral nervous system. J Neurosci Res. 1989 Nov;24(3):451–456. doi: 10.1002/jnr.490240316. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Crewe J. Endotoxin-induced uveitis. Kinetics and phenotype of the inflammatory cell infiltrate and the response of the resident tissue macrophages and dendritic cells in the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995 Sep;36(10):1949–1959. [PubMed] [Google Scholar]
- McMenamin P. G., Crewe J., Morrison S., Holt P. G. Immunomorphologic studies of macrophages and MHC class II-positive dendritic cells in the iris and ciliary body of the rat, mouse, and human eye. Invest Ophthalmol Vis Sci. 1994 Jul;35(8):3234–3250. [PubMed] [Google Scholar]
- Olsson T., Kelic S., Edlund C., Bakhiet M., Höjeberg B., van der Meide P. H., Ljungdahl A., Kristensson K. Neuronal interferon-gamma immunoreactive molecule: bioactivities and purification. Eur J Immunol. 1994 Feb;24(2):308–314. doi: 10.1002/eji.1830240205. [DOI] [PubMed] [Google Scholar]
- Olsson T., Kristensson K., Ljungdahl A., Maehlen J., Holmdahl R., Klareskog L. Gamma-interferon-like immunoreactivity in axotomized rat motor neurons. J Neurosci. 1989 Nov;9(11):3870–3875. doi: 10.1523/JNEUROSCI.09-11-03870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planck S. R., Huang X. N., Robertson J. E., Rosenbaum J. T. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):924–930. [PubMed] [Google Scholar]
- Simmons A., Tscharke D., Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- Tervo K., Tervo T., Eränkö L., Eränkö O., Valtonen S., Cuello A. C. Effect of sensory and sympathetic denervation on substance P immunoreactivity in nerve fibres of the rabbit eye. Exp Eye Res. 1982 Apr;34(4):577–585. doi: 10.1016/0014-4835(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Alm P., Håkanson R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience. 1995 Nov;69(1):297–308. doi: 10.1016/0306-4522(95)00258-k. [DOI] [PubMed] [Google Scholar]
- Whitcup S. M., Rizzo L. V., Lai J. C., Hayashi S., Gazzinelli R., Chan C. C. IL-12 inhibits endotoxin-induced inflammation in the eye. Eur J Immunol. 1996 May;26(5):995–999. doi: 10.1002/eji.1830260506. [DOI] [PubMed] [Google Scholar]
- Yang P., de Vos A. F., Kijlstra A. Macrophages and MHC class II positive cells in the choroid during endotoxin induced uveitis. Br J Ophthalmol. 1997 May;81(5):396–401. doi: 10.1136/bjo.81.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., de Vos A. F., Kijlstra A. Macrophages in the retina of normal Lewis rats and their dynamics after injection of lipopolysaccharide. Invest Ophthalmol Vis Sci. 1996 Jan;37(1):77–85. [PubMed] [Google Scholar]
- Young H. A., Hardy K. J. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995 Oct;58(4):373–381. [PubMed] [Google Scholar]
- de Vos A. F., Klaren V. N., Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994 Oct;35(11):3873–3883. [PubMed] [Google Scholar]
- van der Meide P. H., Borman A. H., Beljaars H. G., Dubbeld M. A., Botman C. A., Schellekens H. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 1989 Winter;8(4):439–449. [PubMed] [Google Scholar]