Abstract
BACKGROUND—Ocular pulse amplitude (OPA) is reduced in normal tension primary open angle glaucoma (NTP) patients when compared with healthy age matched controls (CTL) while increased OPA appears to protect ocular hypertensive patients from visual field loss. If NTP is accompanied by vasospasm, as in roughly half of the primary open angle glaucoma (POAG) population (independent of intraocular pressure, IOP), calcium channel blockers increase OPA and thus stabilise visual fields in these patients. Current glaucoma drugs reduce IOP but do not activate (compromised) ocular perfusion. METHODS—The influence of dorzolamide, a topical carbonic anhydrase inhibitor in standard dosage (three times daily, one eye) on OPA, IOP, blood pressure, and heart rate was investigated in a randomised, prospective, masked clinical trial assessing the acute effects of dorzolamide v placebo before and 2 days after application in 33 cataract patients with (n = 14) and without (n = 19) high tension POAG (HTP) who provided informed consent. RESULTS—Following application of dorzolamide (D) IOP (mm Hg, mean (SEM)) in HTPD (20.2 (0.5)/16.3 (0.5)) and in CTLD (16.0 (0.5)/12.3 (0.5)) was highly significantly (p <0.001) reduced and was significantly (p<0.03) reduced in vehicle (V) treated eyes (HTPV: 20.3 (0.4)/19.0 (0.4)) and CTLV: 15.8 (0.4)/14.9 (0.3)) when compared with respective baseline measurements. OPA (mm Hg) in HTPD (2.1 (0.1)/2.5 (0.1)) and CTLD (2.2 (0.1)/2.6 (0.2)) eyes was significantly (p<0.05) increased and unaffected in vehicle treated eyes when compared with respective baseline measurements. Systemic perfusion variables were also unchanged. CONCLUSION—Dorzolamide increased OPA in HTP and CTL. Drugs stimulating OPA may improve prognosis of POAGs. Keywords: ocular pulse amplitude; primary open angle glaucoma; dorzolamide
Full Text
The Full Text of this article is available as a PDF (107.5 KB).
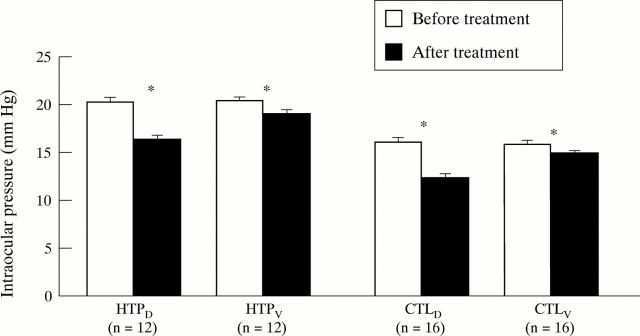
Figure 1 .
Intraocular pressure (IOP) in dorzolamide (D) and vehicle (V) treated eyes of high tension primary open angle glaucoma (HTP) patients (n = 12) and non-glaucomatous controls (CTL, n = 16) before and after drug or vehicle application. Bars represent mean (SEM). *Indicates p<0.05 compared with measurements before drug or vehicle application using the Student's two tailed, paired t test.
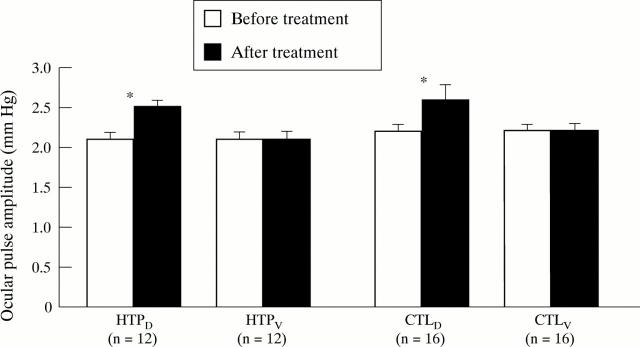
Figure 2 .
Ocular pulse amplitude (OPA) in dorzolamide (D) and vehicle (V) treated eyes of high tension primary open angle glaucoma (HTP) patients (n = 12) and non-glaucomatous controls (CTL, n = 16) before and after drug or vehicle application. Bars represent mean (SEM). *Indicates p<0.05 compared with measurements before drug or vehicle application using the Student's two tailed, paired t test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chidlow G., Nash M. S., Crowhurst C., Bron A. J., Osborne N. N. The ocular blood flow tonograph: a new instrument for the measurement of intraocular pressure in rabbits. Exp Eye Res. 1996 Oct;63(4):463–469. doi: 10.1006/exer.1996.0136. [DOI] [PubMed] [Google Scholar]
- Gambhir S., Inao S., Tadokoro M., Nishino M., Ito K., Ishigaki T., Kuchiwaki H., Yoshida J. Comparison of vasodilatory effect of carbon dioxide inhalation and intravenous acetazolamide on brain vasculature using positron emission tomography. Neurol Res. 1997 Apr;19(2):139–144. doi: 10.1080/01616412.1997.11740787. [DOI] [PubMed] [Google Scholar]
- Gregory D., Sears M., Bausher L., Mishima H., Mead A. Intraocular pressure and aqueous flow are decreased by cholera toxin. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):371–381. [PubMed] [Google Scholar]
- Grunwald J. E., Riva C. E., Stone R. A., Keates E. U., Petrig B. L. Retinal autoregulation in open-angle glaucoma. Ophthalmology. 1984 Dec;91(12):1690–1694. doi: 10.1016/s0161-6420(84)34091-x. [DOI] [PubMed] [Google Scholar]
- Harada K., Miwa A., Yokoyama T., Izawa T., Ogawa N., Jinno Y. Pharmacological analysis of the inhibitory effects of KRN2391 on endothelin-1-induced contraction in isolated large coronary artery of the pig. Gen Pharmacol. 1994 Sep;25(5):935–939. doi: 10.1016/0306-3623(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Harris A., Arend O., Arend S., Martin B. Effects of topical dorzolamide on retinal and retrobulbar hemodynamics. Acta Ophthalmol Scand. 1996 Dec;74(6):569–572. doi: 10.1111/j.1600-0420.1996.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Harris A., Sergott R. C., Spaeth G. L., Katz J. L., Shoemaker J. A., Martin B. J. Color Doppler analysis of ocular vessel blood velocity in normal-tension glaucoma. Am J Ophthalmol. 1994 Nov 15;118(5):642–649. doi: 10.1016/s0002-9394(14)76579-1. [DOI] [PubMed] [Google Scholar]
- Hartenbaum D. The efficacy of dorzolamide, a topical carbonic anhydrase inhibitor, in combination with timolol in the treatment of patients with open-angle glaucoma and ocular hypertension. Clin Ther. 1996 May-Jun;18(3):460–465. doi: 10.1016/s0149-2918(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Tanaka Y., Hirose Y., Kume N., Iwama T., Miyake Y., Ishida Y., Matsuura H., Miyake Y., Nishimura T. Vasoreactive effect of acetazolamide as a function of time with sequential PET 15O-water measurement. Nucl Med Commun. 1996 Dec;17(12):1047–1051. doi: 10.1097/00006231-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Heijl A., Strahlman E., Sverrisson T., Brinchman-Hansen O., Puustjärvi T., Tipping R. A comparison of dorzolamide and timolol in patients with pseudoexfoliation and glaucoma or ocular hypertension. Ophthalmology. 1997 Jan;104(1):137–142. doi: 10.1016/s0161-6420(97)30348-0. [DOI] [PubMed] [Google Scholar]
- Higginbotham E. J., Kass M. A., Lippa E. A., Batenhorst R. L., Panebianco D. L., Wilensky J. T. MK-927: a topical carbonic anhydrase inhibitor. Dose response and duration of action. Arch Ophthalmol. 1990 Jan;108(1):65–68. doi: 10.1001/archopht.1990.01070030071031. [DOI] [PubMed] [Google Scholar]
- Kiel J. W. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994 May;58(5):529–543. doi: 10.1006/exer.1994.1047. [DOI] [PubMed] [Google Scholar]
- Kiel J. W., Shepherd A. P. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1992 Jul;33(8):2399–2410. [PubMed] [Google Scholar]
- Kitazawa Y., Shirai H., Go F. J. The effect of Ca2(+) -antagonist on visual field in low-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 1989;227(5):408–412. doi: 10.1007/BF02172889. [DOI] [PubMed] [Google Scholar]
- Langham M. E., To'Mey K. F. A clinical procedure for the measurements of the ocular pulse-pressure relationship and the ophthalmic arterial pressure. Exp Eye Res. 1978 Jul;27(1):17–25. doi: 10.1016/0014-4835(78)90049-0. [DOI] [PubMed] [Google Scholar]
- Lippa E. A., Aasved H., Airaksinen P. J., Alm A., Bertelsen T., Calissendorff B., Dithmer O., Eriksson L. O., Gustad L., Høvding G. Multiple-dose, dose-response relationship for the topical carbonic anhydrase inhibitor MK-927. Arch Ophthalmol. 1991 Jan;109(1):46–49. doi: 10.1001/archopht.1991.01080010048030. [DOI] [PubMed] [Google Scholar]
- Lippa E. A., von Denffer H. A., Hofmann H. M., Brunner-Ferber F. L. Local tolerance and activity of MK-927, a novel topical carbonic anhydrase inhibitor. Arch Ophthalmol. 1988 Dec;106(12):1694–1696. doi: 10.1001/archopht.1988.01060140866029. [DOI] [PubMed] [Google Scholar]
- Martin X. D., Rabineau P. A. Vasoconstrictive effect of topical timolol on human retinal arteries. Graefes Arch Clin Exp Ophthalmol. 1989;227(6):526–530. doi: 10.1007/BF02169445. [DOI] [PubMed] [Google Scholar]
- Nagataki S., Brubaker R. F. Early effect of epinephrine on aqueous formation in the normal human eye. Ophthalmology. 1981 Mar;88(3):278–282. doi: 10.1016/s0161-6420(81)35039-8. [DOI] [PubMed] [Google Scholar]
- Netland P. A., Chaturvedi N., Dreyer E. B. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am J Ophthalmol. 1993 May 15;115(5):608–613. doi: 10.1016/s0002-9394(14)71458-8. [DOI] [PubMed] [Google Scholar]
- Nighoghossian N., Berthezene Y., Meyer R., Cinotti L., Adeleine P., Philippon B., Froment J. C., Trouillas P. Assessment of cerebrovascular reactivity by dynamic susceptibility contrast-enhanced MR imaging. J Neurol Sci. 1997 Aug;149(2):171–176. doi: 10.1016/s0022-510x(97)05393-8. [DOI] [PubMed] [Google Scholar]
- Nyborg N. C., Prieto D., Benedito S., Nielsen P. J. Endothelin-1-induced contraction of bovine retinal small arteries is reversible and abolished by nitrendipine. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):27–31. [PubMed] [Google Scholar]
- Pfeiffer N., Hennekes R., Lippa E. A., Grehn F., Garus H., Brunner-Ferber F. L. A single dose of the topical carbonic anhydrase inhibitor MK-927 decreases IOP in patients. Br J Ophthalmol. 1990 Jul;74(7):405–408. doi: 10.1136/bjo.74.7.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillunat L. E., Stodtmeister R., Marquardt R., Mattern A. Ocular perfusion pressures in different types of glaucoma. Int Ophthalmol. 1989 Jan;13(1-2):37–42. doi: 10.1007/BF02028635. [DOI] [PubMed] [Google Scholar]
- Postiglione A., Bobkiewicz T., Vinholdt-Pedersen E., Lassen N. A., Paulson O. B., Barry D. I. Cerebrovascular effects of angiotensin converting enzyme inhibition involve large artery dilatation in rats. Stroke. 1991 Nov;22(11):1363–1368. doi: 10.1161/01.str.22.11.1363. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Hohman R. M., Sanchez R., Addicks E. M. Optic nerve head blood flow in chronic experimental glaucoma. Arch Ophthalmol. 1985 Jul;103(7):956–962. doi: 10.1001/archopht.1985.01050070082035. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y., Wistrand P. J., Brechue W. F. Membrane-associated CA activity in the eye of the CA II-deficient mouse. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2577–2584. [PubMed] [Google Scholar]
- Rojanapongpun P., Drance S. M., Morrison B. J. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993 Jan;77(1):25–29. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. G., Mittag T. W., Pavlovic S., Hessemer V. Influence of physical exercise and nifedipine on ocular pulse amplitude. Graefes Arch Clin Exp Ophthalmol. 1996 Aug;234(8):527–532. doi: 10.1007/BF00184863. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., Rückmann A. V., Mittag T. W., Hessemer V., Pillunat L. E. Reduced ocular pulse amplitude in low tension glaucoma is independent of vasospasm. Eye (Lond) 1997;11(Pt 4):485–488. doi: 10.1038/eye.1997.131. [DOI] [PubMed] [Google Scholar]
- Schmidt K. G., von Rückmann A., Mittag T. W. Okuläre Pulsamplitude bei okulärer Hypertension und verschiedenen Glaukomformen. Ophthalmologica. 1998;212(1):5–10. doi: 10.1159/000027250. [DOI] [PubMed] [Google Scholar]
- Serle J. B., Lustgarten J. S., Lippa E. A., Camras C. B., Panebianco D. L., Podos S. M. MK-927, a topical carbonic anhydrase inhibitor. Dose response and reproducibility. Arch Ophthalmol. 1990 Jun;108(6):838–841. doi: 10.1001/archopht.1990.01070080080039. [DOI] [PubMed] [Google Scholar]
- Spielmann A. Nystagmus. Curr Opin Ophthalmol. 1994 Oct;5(5):20–24. [PubMed] [Google Scholar]
- Townsend D. J., Brubaker R. F. Immediate effect of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci. 1980 Mar;19(3):256–266. [PubMed] [Google Scholar]
- Trew D. R., Smith S. E. Postural studies in pulsatile ocular blood flow: II. Chronic open angle glaucoma. Br J Ophthalmol. 1991 Feb;75(2):71–75. doi: 10.1136/bjo.75.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk E. M. The ciliary vasculature and its perturbation with drugs and surgery. Trans Am Ophthalmol Soc. 1988;86:794–841. [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Serle J. B., Podos S. M., Sugrue M. F. MK-507 (L-671,152), a topically active carbonic anhydrase inhibitor, reduces aqueous humor production in monkeys. Arch Ophthalmol. 1991 Sep;109(9):1297–1299. doi: 10.1001/archopht.1991.01080090123036. [DOI] [PubMed] [Google Scholar]
- Williamson T. H., Harris A. Ocular blood flow measurement. Br J Ophthalmol. 1994 Dec;78(12):939–945. doi: 10.1136/bjo.78.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]