Abstract
BACKGROUND/AIMS—A checkpoint mechanism in late G1, whose regulation via loss of retinoblastoma protein (pRB) or p16, or overexpression of cyclin D1 or cyclin dependent kinase 4 (CDK4), has been proposed to constitute a common pathway to malignancy. The aims of this study were (a) to compare markers of cell cycle G1-S phase transition in an intraocular tumour with known pRB deficiency (retinoblastoma) and compare it with one with an apparently functional pRB (uveal melanoma); (b) to determine if one of these markers may have a role in the pathogenesis of uveal melanoma; and (c) to determine if there is a difference in cell cycle marker expression following treatment of uveal melanoma and retinoblastoma. METHODS—90 eyes were enucleated from 89 patients for retinoblastoma (n=24) or for choroidal or ciliary body melanoma (n=66). Conventional paraffin sections were assessed for cell type and degree of differentiation. Additional slides were investigated applying standard immunohistochemical methods with antibodies specific for cyclin D1 protein, pRB, p53, p21, p16, BCL-2, and MIB-1. RESULTS—Cyclin D1 protein and pRB were negative in retinoblastoma using the applied antibodies. In contrast, cyclin D1 protein expression was observed in 65% of uveal melanomas; a positive correlation between cyclin D1 cell positivity and tumour cell type, location, growth fraction, as well as with pRB positivity was observed. p53, p21, and p16 could be demonstrated in both tumours. An inverse relation between p53 and p21 expression was demonstrated in most choroidal melanomas and in some retinoblastomas. Apart from a decrease in the growth fractions of the tumours as determined by MIB-1, a significant difference in the expression of G1-S phase transition markers in vital areas of uveal melanoma and retinoblastoma following treatment with radiotherapy and/or chemotherapy was not observed. CONCLUSION—Retinoblastomas and uveal melanomas, two tumours of differing pRB status, differ also in their immunohistochemical pattern for markers of the G1-S phase transition of the cell cycle. The results of the present study support the concept of (a) an autoregulatory loop between pRB and cyclin D1 in tumours with a functional pRB and the disruption of this loop in the presence of pRB mutation, as well as (b) a checkpoint mechanism in late G1, whose regulation via loss of p16 or pRB, or overexpression of cyclin D1 constitutes a common pathway to malignancy. Further, the results raise the possibility of cyclin D1 overexpression having a role in the pathogenesis of uveal melanoma. Keywords: cyclin D1; retinoblastoma protein; antigens; antibodies; bipolar cells; uveal melanoma; retinoblastoma
Full Text
The Full Text of this article is available as a PDF (308.0 KB).
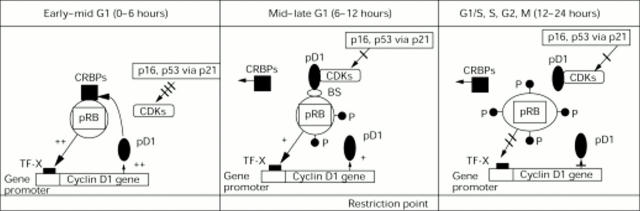
Figure 1 .
Interactions between cyclin D1 gene and protein (pD1) and retinoblastoma protein (pRB) as well as between the cyclin dependent kinases (CDKs) and their inhibitors (p16 and p21 via p53) during the cell cycle (modified from Lukas et al55). During early to mid G1, the concentrations of pD1 are low. pRB is hypophosphorylated and has a stimulatory activity on the transcription of the cyclin D1 gene. At this time, the retinoblastoma "pockets" are occupied by "cellular retinoblastoma binding proteins" (CRBPs), which are potent cell cycle stimulators when released later from the pRB pockets. The CDKs are inhibited by the CDKIs, including p16 and p21, the latter being stimulated by p53. The combination of hypophosphorylated pRB, the pRB bound CRBPs, and the inhibitory effects of the CDKIs contribute to cell cycle arrest at the restriction point (R point) in the mid to late G1 phase. The concentrations of pD1 increase and eventually are sufficient to combine with the CDKs, overriding the inhibitory activity of the CDKIs and resulting in (a) the displacement of the CRBPs from the RB pockets, (b) phosphorylation of the pRB, causing a change in the configuration of the pRB molecule. These alterations stimulate cell progression into the S phase, as well as a decrease in the transcriptional stimulus of the cyclin D1 gene. The concentration of pD1, consequentially, decreases during the S, G2, and M phases. TF-X represents a proposed transcription factor55 and BS, a possible binding site or molecule through which cyclin D1 indirectly interacts with pRB.
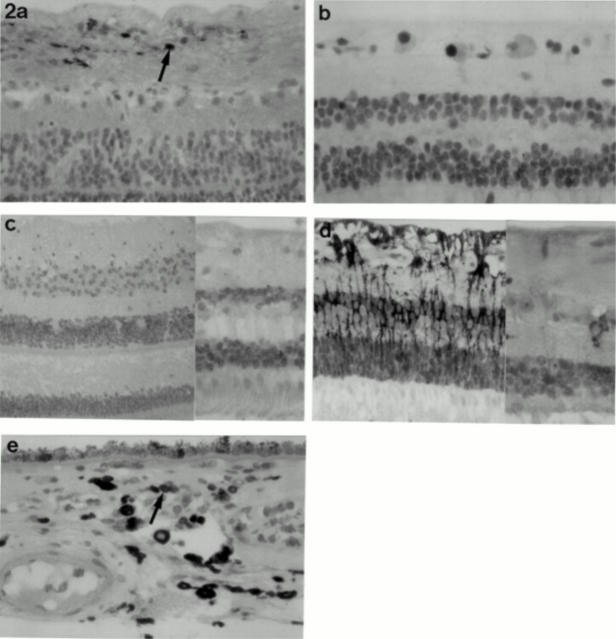
Figure 2 .
Normal retina with (a) cyclin D1 staining in occasional ganglion cells (arrow) (×20 objective); (b) p53 positivity in scattered ganglion cells (×40 objective); (c) granular p21 staining in all cell layers of the retina (×20 and ×40 objective); (d) BCL-2 positivity in glial cells and, at the higher magnification, in occasional ganglion cells (×20 and ×40 objective). (e) Normal choroid with scattered BCL-2 positive reactive lymphocytes (arrow) (×40 objective); occasional melanocytes demonstrated weak BCL-2 positivity following bleaching (not shown).
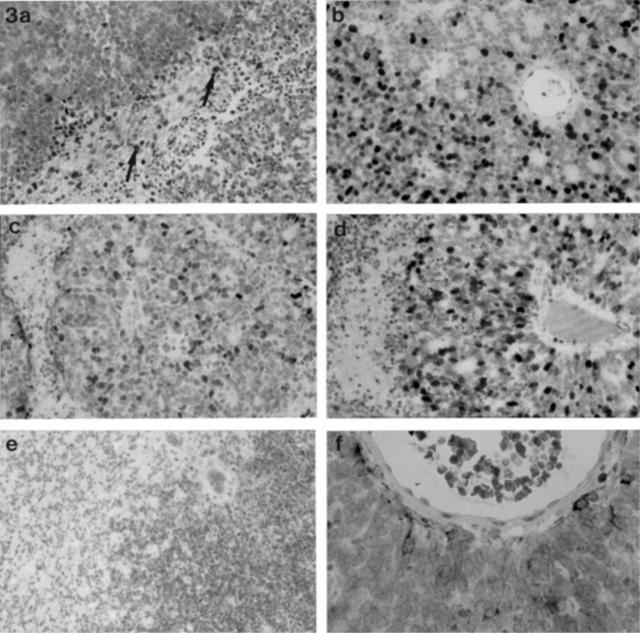
Figure 3 .
(a) Cyclin D1 positivity in reactive glial cells (arrow) within retinoblastoma; the tumour cells are negative (×20 objective). (b) The degree of differentiation within the retinoblastomas is reflected by the corresponding MIB-1 growth fractions (×20 objective); (c) p53; and (d) p21 positivity surrounding a pseudorosette where staining of these markers is present in all "zones" (×40 objective); (e) p16 staining in poorly and moderately differentiated tumours areas within a retinoblastoma (×20 objective). (f) Perivascular glial cells positive for BCL-2 in a retinoblastoma; the tumour cells are negative (×40 objective).
Figure 4 .
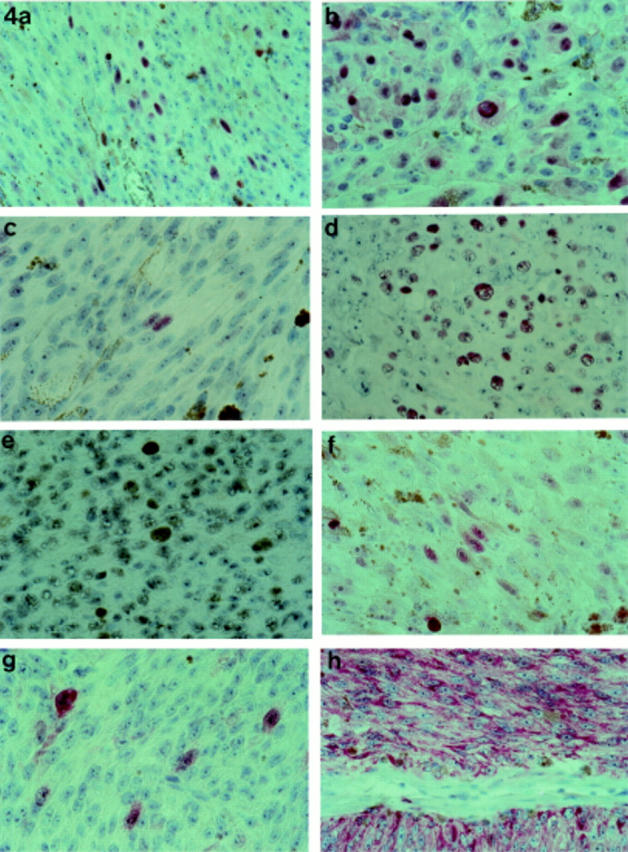
Cyclin D1 positivity in a spindle (a) and epithelioid (b) choroidal melanoma (×20 objective). pRB positivity in a spindle (c) and epithelioid (d) choroidal melanoma (×20 objective). (e) Clear p53 positivity in choroidal melanoma (arrow) (×20 objective). (f) p21 staining in occasional tumour cells of a mixed cell choroidal melanoma (×20 objective). (g) p16 positivity in scattered tumour cells of a mixed cell choroidal melanoma (×40 objective). (h) BCL-2 staining in a spindle choroidal melanoma (×20 objective).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartkova J., Lukas J., Müller H., Lützhøft D., Strauss M., Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994 May 1;57(3):353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Strauss M., Bartek J. Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene. 1995 Feb 16;10(4):775–778. [PubMed] [Google Scholar]
- Basolo F., Pollina L., Fontanini G., Fiore L., Pacini F., Baldanzi A. Apoptosis and proliferation in thyroid carcinoma: correlation with bcl-2 and p53 protein expression. Br J Cancer. 1997;75(4):537–541. doi: 10.1038/bjc.1997.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltinger C. P., White P. S., Sulman E. P., Maris J. M., Brodeur G. M. No CDKN2 mutations in neuroblastomas. Cancer Res. 1995 May 15;55(10):2053–2055. [PubMed] [Google Scholar]
- Buckley M. F., Sweeney K. J., Hamilton J. A., Sini R. L., Manning D. L., Nicholson R. I., deFazio A., Watts C. K., Musgrove E. A., Sutherland R. L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993 Aug;8(8):2127–2133. [PubMed] [Google Scholar]
- Char D. H., Huhta K., Waldman F. DNA cell cycle studies in uveal melanoma. Am J Ophthalmol. 1989 Jan 15;107(1):65–72. doi: 10.1016/0002-9394(89)90817-9. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Costa M. J., Walls J., Ames P., Roth L. M. Transformation in recurrent ovarian granulosa cell tumors: Ki67 (MIB-1) and p53 immunohistochemistry demonstrates a possible molecular basis for the poor histopathologic prediction of clinical behavior. Hum Pathol. 1996 Mar;27(3):274–281. doi: 10.1016/s0046-8177(96)90069-6. [DOI] [PubMed] [Google Scholar]
- Doglioni C., Pelosio P., Laurino L., Macri E., Meggiolaro E., Favretti F., Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996 Jul;179(3):248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Fuchs U., Kivelä T., Summanen P., Immonen I., Tarkkanen A. An immunohistochemical and prognostic analysis of cytokeratin expression in malignant uveal melanoma. Am J Pathol. 1992 Jul;141(1):169–181. [PMC free article] [PubMed] [Google Scholar]
- Gao X., Chen Y. Q., Wu N., Grignon D. J., Sakr W., Porter A. T., Honn K. V. Somatic mutations of the WAF1/CIP1 gene in primary prostate cancer. Oncogene. 1995 Oct 5;11(7):1395–1398. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Golusiński W., Olofsson J., Szmeja Z., Majewski P. p53, PCNA and Ki67 in laryngeal cancer. Pol J Pathol. 1996;47(4):175–182. [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995 Apr;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hirama T., Koeffler H. P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995 Aug 1;86(3):841–854. [PubMed] [Google Scholar]
- Horsman D. E., Sroka H., Rootman J., White V. A. Monosomy 3 and isochromosome 8q in a uveal melanoma. Cancer Genet Cytogenet. 1990 Apr;45(2):249–253. doi: 10.1016/0165-4608(90)90090-w. [DOI] [PubMed] [Google Scholar]
- Horsman D. E., White V. A. Cytogenetic analysis of uveal melanoma. Consistent occurrence of monosomy 3 and trisomy 8q. Cancer. 1993 Feb 1;71(3):811–819. doi: 10.1002/1097-0142(19930201)71:3<811::aid-cncr2820710325>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Janssen K., Kuntze J., Busse H., Schmid K. W. p53 oncoprotein overexpression in choroidal melanoma. Mod Pathol. 1996 Mar;9(3):267–272. [PubMed] [Google Scholar]
- Jay V., Yi Q., Hunter W. S., Zielenska M. Expression of bcl-2 in uveal malignant melanoma. Arch Pathol Lab Med. 1996 May;120(5):497–498. [PubMed] [Google Scholar]
- Jensen O. A. Malignant melanomas of the human uvea: 25-year follow-up of cases in Denmark, 1943--1952. Acta Ophthalmol (Copenh) 1982 Apr;60(2):161–182. doi: 10.1111/j.1755-3768.1982.tb08371.x. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr The genetics of childhood cancer. Cancer. 1975 Mar;35(3 Suppl):1022–1026. doi: 10.1002/1097-0142(197503)35:3+<1022::aid-cncr2820350726>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kock E., Naeser P. Retinoblastoma in Sweden 1958--1971. A clinical and histopathological study. Acta Ophthalmol (Copenh) 1979 Jun;57(3):344–350. doi: 10.1111/j.1755-3768.1979.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Ku S., Talpaz M. Abnormalities in the PRAD1 (CYCLIN D1/BCL-1) oncogene are frequent in cervical and vulvar squamous cell carcinoma cell lines. Cancer. 1995 Jan 15;75(2):584–590. doi: 10.1002/1097-0142(19950115)75:2<584::aid-cncr2820750223>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lukas J., Müller H., Bartkova J., Spitkovsky D., Kjerulff A. A., Jansen-Dürr P., Strauss M., Bartek J. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1. J Cell Biol. 1994 May;125(3):625–638. doi: 10.1083/jcb.125.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney M. C., Burnett W. S., Majerovics A., Tanenbaum H. The epidemiology of ophthalmic malignancies in New York State. Ophthalmology. 1990 Sep;97(9):1143–1147. doi: 10.1016/s0161-6420(90)32445-4. [DOI] [PubMed] [Google Scholar]
- Mate J. L., Ariza A., Aracil C., López D., Isamat M., Pérez-Piteira J., Navas-Palacios J. J. Cyclin D1 overexpression in non-small cell lung carcinoma: correlation with Ki67 labelling index and poor cytoplasmic differentiation. J Pathol. 1996 Dec;180(4):395–399. doi: 10.1002/(SICI)1096-9896(199612)180:4<395::AID-PATH688>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Foster W. D., Zimmerman L. E., Gamel J. W. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983 Oct;96(4):502–509. doi: 10.1016/s0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Veelen N., Hart A., Loftus B., Wientjens E., Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995 Mar 1;55(5):975–978. [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Mooy C. M., Luyten G. P., de Jong P. T., Luider T. M., Stijnen T., van de Ham F., van Vroonhoven C. C., Bosman F. T. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am J Pathol. 1995 Oct;147(4):1097–1104. [PMC free article] [PubMed] [Google Scholar]
- Mooy C. M., de Jong P. T., Van der Kwast T. H., Mulder P. G., Jager M. J., Ruiter D. J. Ki-67 immunostaining in uveal melanoma. The effect of pre-enucleation radiotherapy. Ophthalmology. 1990 Oct;97(10):1275–1280. doi: 10.1016/s0161-6420(90)32420-x. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Naitoh H., Shibata J., Kawaguchi A., Kodama M., Hattori T. Overexpression and localization of cyclin D1 mRNA and antigen in esophageal cancer. Am J Pathol. 1995 May;146(5):1161–1169. [PMC free article] [PubMed] [Google Scholar]
- Nawa G., Ueda T., Mori S., Yoshikawa H., Fukuda H., Ishiguro S., Funai H., Uchida A. Prognostic significance of Ki67 (MIB1) proliferation index and p53 over-expression in chondrosarcomas. Int J Cancer. 1996 Apr 22;69(2):86–91. doi: 10.1002/(SICI)1097-0215(19960422)69:2<86::AID-IJC3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Nork T. M., Poulsen G. L., Millecchia L. L., Jantz R. G., Nickells R. W. p53 regulates apoptosis in human retinoblastoma. Arch Ophthalmol. 1997 Feb;115(2):213–219. doi: 10.1001/archopht.1997.01100150215011. [DOI] [PubMed] [Google Scholar]
- Nork T. M., Schwartz T. L., Doshi H. M., Millecchia L. L. Retinoblastoma. Cell of origin. Arch Ophthalmol. 1995 Jun;113(6):791–802. doi: 10.1001/archopht.1995.01100060117046. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Ohta M., Berd D., Shimizu M., Nagai H., Cotticelli M-G, Mastrangelo M., Shields J. A., Shields C. L., Croce C. M., Huebner K. Deletion mapping of chromosome region 9p21-p22 surrounding the CDKN2 locus in melanoma. Int J Cancer. 1996 Mar 15;65(6):762–767. doi: 10.1002/(SICI)1097-0215(19960315)65:6<762::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. M., Helbing A., Ott G., Bartek J., Fischer L., Dürr A., Kreipe H., Müller-Hermelink H. K. bcl-1 rearrangement and cyclin D1 protein expression in mantle cell lymphoma. J Pathol. 1996 Jul;179(3):238–242. doi: 10.1002/(SICI)1096-9896(199607)179:3<238::AID-PATH566>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Otterson G. A., Khleif S. N., Chen W., Coxon A. B., Kaye F. J. CDKN2 gene silencing in lung cancer by DNA hypermethylation and kinetics of p16INK4 protein induction by 5-aza 2'deoxycytidine. Oncogene. 1995 Sep 21;11(6):1211–1216. [PubMed] [Google Scholar]
- Otterson G. A., Kratzke R. A., Coxon A., Kim Y. W., Kaye F. J. Absence of p16INK4 protein is restricted to the subset of lung cancer lines that retains wildtype RB. Oncogene. 1994 Nov;9(11):3375–3378. [PubMed] [Google Scholar]
- Pendergrass T. W., Davis S. Incidence of retinoblastoma in the United States. Arch Ophthalmol. 1980 Jul;98(7):1204–1210. doi: 10.1001/archopht.1980.01020040056003. [DOI] [PubMed] [Google Scholar]
- Piris M. A., Villuendas R., Martinez J. C., Sanchez-Beato M., Orradre J. L., Mateo M. S., Martinez P. p53 expression in non-Hodgkin's lymphomas: a marker of p53 inactivation? Leuk Lymphoma. 1995 Mar;17(1-2):35–42. doi: 10.3109/10428199509051701. [DOI] [PubMed] [Google Scholar]
- Prescher G., Bornfeld N., Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990 Nov 21;82(22):1765–1769. doi: 10.1093/jnci/82.22.1765. [DOI] [PubMed] [Google Scholar]
- Prescher G., Bornfeld N., Horsthemke B., Becher R. Chromosomal aberrations defining uveal melanoma of poor prognosis. Lancet. 1992 Mar 14;339(8794):691–692. [PubMed] [Google Scholar]
- Prokocimer M., Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994 Oct 15;84(8):2391–2411. [PubMed] [Google Scholar]
- Sakaguchi M., Fujii Y., Hirabayashi H., Yoon H. E., Komoto Y., Oue T., Kusafuka T., Okada A., Matsuda H. Inversely correlated expression of p16 and Rb protein in non-small cell lung cancers: an immunohistochemical study. Int J Cancer. 1996 Feb 8;65(4):442–445. doi: 10.1002/(SICI)1097-0215(19960208)65:4<442::AID-IJC8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sanders B. M., Draper G. J., Kingston J. E. Retinoblastoma in Great Britain 1969-80: incidence, treatment, and survival. Br J Ophthalmol. 1988 Aug;72(8):576–583. doi: 10.1136/bjo.72.8.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling H., Sehu K. W., Lee W. R. A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci. 1997 Sep;38(10):2081–2092. [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Silvestrini R., Veneroni S., Daidone M. G., Benini E., Boracchi P., Mezzetti M., Di Fronzo G., Rilke F., Veronesi U. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994 Apr 6;86(7):499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- Sisley K., Cottam D. W., Rennie I. G., Parsons M. A., Potter A. M., Potter C. W., Rees R. C. Non-random abnormalities of chromosomes 3, 6, and 8 associated with posterior uveal melanoma. Genes Chromosomes Cancer. 1992 Oct;5(3):197–200. doi: 10.1002/gcc.2870050304. [DOI] [PubMed] [Google Scholar]
- Sisley K., Rennie I. G., Cottam D. W., Potter A. M., Potter C. W., Rees R. C. Cytogenetic findings in six posterior uveal melanomas: involvement of chromosomes 3, 6, and 8. Genes Chromosomes Cancer. 1990 Sep;2(3):205–209. doi: 10.1002/gcc.2870020307. [DOI] [PubMed] [Google Scholar]
- Speicher M. R., Prescher G., du Manoir S., Jauch A., Horsthemke B., Bornfeld N., Becher R., Cremer T. Chromosomal gains and losses in uveal melanomas detected by comparative genomic hybridization. Cancer Res. 1994 Jul 15;54(14):3817–3823. [PubMed] [Google Scholar]
- Tobal K., Warren W., Cooper C. S., McCartney A., Hungerford J., Lightman S. Increased expression and mutation of p53 in choroidal melanoma. Br J Cancer. 1992 Nov;66(5):900–904. doi: 10.1038/bjc.1992.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T., Tsunoda S., Sakaki T., Konishi N., Hiasa Y., Nakamura M. Alterations of retinoblastoma, p53, p16(CDKN2), and p15 genes in human astrocytomas. Cancer. 1996 Jul 15;78(2):287–293. doi: 10.1002/(SICI)1097-0142(19960715)78:2<287::AID-CNCR15>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- Yang E., Korsmeyer S. J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996 Jul 15;88(2):386–401. [PubMed] [Google Scholar]
- Yeager T., Stadler W., Belair C., Puthenveettil J., Olopade O., Reznikoff C. Increased p16 levels correlate with pRb alterations in human urothelial cells. Cancer Res. 1995 Feb 1;55(3):493–497. [PubMed] [Google Scholar]
- Yuge K., Nakajima M., Uemura Y., Miki H., Uyama M., Tsubura A. Immunohistochemical features of the human retina and retinoblastoma. Virchows Arch. 1995;426(6):571–575. doi: 10.1007/BF00192111. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. Specific fine chromosomal defects in cancer: an overview. Hum Pathol. 1981 Jun;12(6):503–515. doi: 10.1016/s0046-8177(81)80064-0. [DOI] [PubMed] [Google Scholar]
- Zukerberg L. R., Benedict W. F., Arnold A., Dyson N., Harlow E., Harris N. L. Expression of the retinoblastoma protein in low-grade B-cell lymphoma: relationship to cyclin D1. Blood. 1996 Jul 1;88(1):268–276. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]