Abstract
AIMS/BACKGROUND—The authors have developed transgenic mouse strains at the Arizona Cancer Center using a tyrosinase promoter to target expression of the mutated T24 Ha-ras gene in melanin producing cells. Histopathology and electron microscopy (EM) were performed to characterise the intraocular tumours observed phenotypically. METHODS—Transgenic TPras mice (n=8) and normal, age matched control mice (n=6) were sacrificed at 3 weeks, 6 weeks, 7 weeks, 4 months, 5 months, 9 months, and 11 months. Six were processed in formalin for light microscopic examination and eight in a glutaraldehyde/formalin solution for electron microscopic examination. RESULTS—Six of the TPras mice were found to have bilateral pigmented melanocytic/RPE proliferations of the uveal tract. The cytological characteristics of the tumours included low nuclear to cytoplasmic ratios (N:C ratios), bland nuclei, and abundant intracytoplasmic melanin. By EM two populations of cells were identified, including spindle-shaped cells with round to oval melanin granules and cuboidal cells with apically located, cigar-shaped, melanin granules, and basement membrane formation. A 3 week and an 11 month old TPras mouse had a higher grade, bilateral, melanocytic proliferation of the uveal tract which, although not metastatic, was morphologically melanoma. Cytological features included increased N:C ratios, nuclear pleomorphism, and prominent nucleoli. The uveal tract was normal in both eyes in all of the control animals. CONCLUSION—Pigmented intraocular tumours developed in transgenic strains of mice that express a mutated Ha-ras gene in melanin producing cells. The morphology was most consistent with a melanoma in two of the mice and a benign melanocytic/RPE proliferation in the remaining mice. Keywords: histopathology; melanoma model; transgenic mice; uveal melanoma
Full Text
The Full Text of this article is available as a PDF (299.4 KB).
Figure 1 .
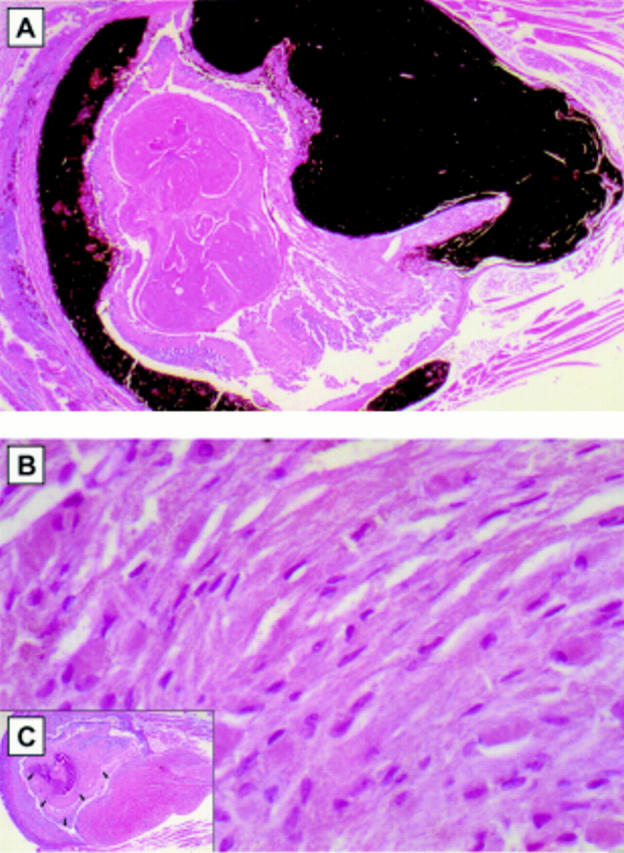
(A) Light micrograph of melanocytic hamartoma in the uveal tract of TPras transgenic mice. The melanocytic proliferation is seen to fill the entire uveal tract. Cells are deeply pigmented and morphological features are not readily discernible (haematoxylin and eosin, original magnification ×10). (B) High power light micrograph of the bleached specimen demonstrates the cytological characteristics of the melanocytic/RPE hamartoma with oval nuclei with bland nuclear characteristics (haematoxylin and eosin, original magnification ×10). Inset (C): Bleached specimen demonstrates a proliferation of plump polyhedral to spindle-shaped cells diffusely filling the uveal tract.
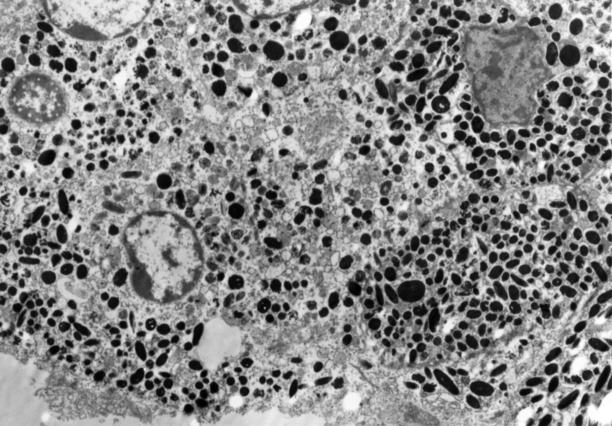
Figure 2 .
An electron micrograph of a melanocytic hamartoma in the uveal tract of TPras transgenic mice. Both spindle cells and cuboidal cells with oval nuclei containing marginated heterochromatin are identified. Intracytoplasmic membranous vesicles, premelanosomes, phagosomes, and cigar-shaped and oval melanin granules are identified. The melanosomes measure between 0.4 and 1.50 µm. Atypical nuclear features are identified in one cell (lower left) which contains a prominent nucleolus (original magnification ×5510).
Figure 3 .
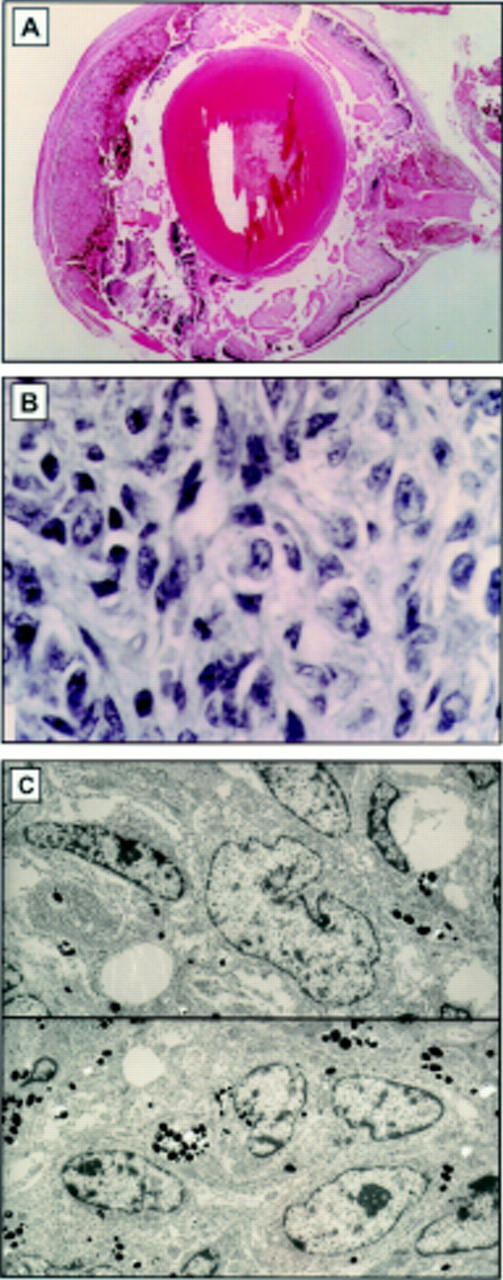
(A) Light micrograph of melanoma in the uveal tract of TPras transgenic mice. The melanocytic proliferation is seen to fill the entire uveal tract. Cells are moderately pigmented and morphological features are not readily discernible (haematoxylin and eosin, original magnification ×10). (B) High power light micrograph of the bleached specimen demonstrates a proliferation of pleomorphic, spindle-shaped cells. The cytological characteristics of the malignant melanoma in the 3 week TPras mouse are demonstrated. (C) Ultrastructurally, the cells are spindle shaped with oval to round nuclei, increased nuclear to cytoplasmic ratios, marginated chromatin, irregular nuclear envelope, and prominent nucleoli. Intracytoplasmic melanin granules are demonstrated (haematoxylin and eosin, original magnification ×160).
Figure 4 .
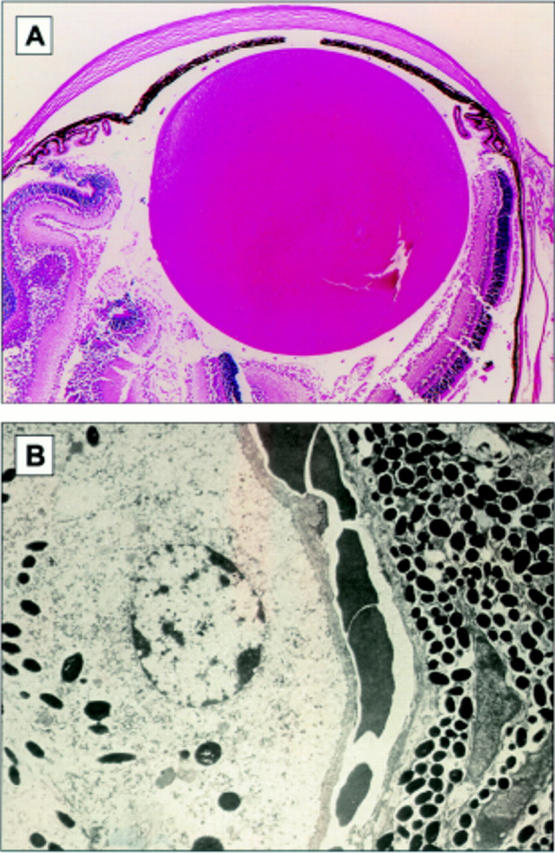
(A) Photomicrograph of the anterior uveal tract in one of the control (negative littermate) mice. The iris ciliary body and choroid are pigmented and normal in structure. There is no evidence of infiltration hyperplasia or hypertrophy of the uveal tract (original magnification ×44). (B) Electron micrograph demonstrating the retinal/choroidal junction in one of the control mice. Choroidal melanocytes are spindle-shaped cells with oval nuclei containing a bland chromatin pattern. Intracytoplasmic round and oval melanin granules are identified. The melanosomes measure 0.50 µm by 0.75 µm. The RPE is composed of cuboidal cells with oval nuclei containing marginated heterochromatin. There is apical to basal polarity and basement membrane formation. Intracytoplasmic rough endoplasmic phagosomes and membranous vesicles are identified. Intracytoplasmic cigar-shaped and oval melanin granules are identified and measure 0.44 µm by 1.58 µm (original magnification ×9180).
Figure 5 .
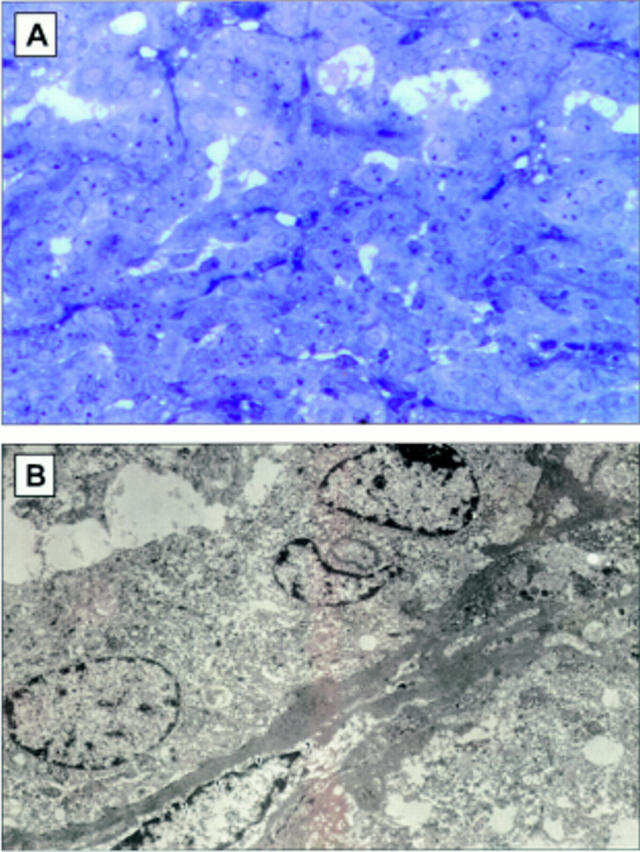
(A) Light micrograph demonstrating a retinal pigment epithelial adenomatous proliferation found in the uveal tract of a normal control litter mate. (B) Histopathologically, this was characterised as a melanotic proliferation of RPE between the retina and the choroid. The cells were noted to form tubuloacinar configurations (haematoxylin and eosin, original magnification ×160).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. M., Shadduck J. A., Liu H. S., Sunderman F. W., Jr, Wagoner M. D., Dohlman H. G., Papale J. J. Animal models for the study of uveal melanoma. Int Ophthalmol Clin. 1980 Summer;20(2):143–160. [PubMed] [Google Scholar]
- Albert D. M., Shadduck J. A., Liu H. S., Sunderman F. W., Jr, Wagoner M. D., Dohlman H. G., Papale J. J. Animal models for the study of uveal melanoma. Int Ophthalmol Clin. 1980 Summer;20(2):143–160. [PubMed] [Google Scholar]
- Albert D. M., Wagoner M. D., Moazed K., Kimball G. P., Gonder J. R. Heterotransplantation of human choroidal melanoma into the athymic "nude" mouse. Invest Ophthalmol Vis Sci. 1980 May;19(5):555–559. [PubMed] [Google Scholar]
- Anand R., Ma D., Alizadeh H., Comerford S. A., Sambrook J. F., Gething M. J., McLean I. W., Niederkorn J. Y. Characterization of intraocular tumors arising in transgenic mice. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3533–3539. [PubMed] [Google Scholar]
- BENSON W. R. Intraocular tumor after ethionine and N-2-fluorenylacetamide. Arch Pathol. 1962 May;73:404–406. [PubMed] [Google Scholar]
- BLODI F. C., REULING F. H., SORNSON E. T. PSEUDOMELANOCYTOMA AT THE OPTIC NERVEHEAD: AN ADENOMA OF THE RETINAL PIGMENT EPITHELIUM. Arch Ophthalmol. 1965 Mar;73:353–355. doi: 10.1001/archopht.1965.00970030355011. [DOI] [PubMed] [Google Scholar]
- Bailleul B., Lang J., Wilkie N., Balmain A. A human T24 Ha-ras cassette suitable for expression studies in eukaryotic cells. Nucleic Acids Res. 1988 Jan 11;16(1):359–359. doi: 10.1093/nar/16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr C. C., Zimmerman L. E., Curtin V. T., Font R. L. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms. A recently recognized syndrome. Arch Ophthalmol. 1982 Feb;100(2):249–255. doi: 10.1001/archopht.1982.01030030251003. [DOI] [PubMed] [Google Scholar]
- Beermann F., Schmid E., Schütz G. Expression of the mouse tyrosinase gene during embryonic development: recapitulation of the temporal regulation in transgenic mice. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2809–2813. doi: 10.1073/pnas.89.7.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M., Klein-Szanto A., Porter S., Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Winkelmann R. K. Acanthosis nigricans: a study of 90 cases. Medicine (Baltimore) 1968 Jan;47(1):33–51. [PubMed] [Google Scholar]
- CURTH H. O., HILBERG A. W., MACHACEK G. F. The site and histology of the cancer associated with malignant acanthosis nigricans. Cancer. 1962 Mar-Apr;15:364–382. doi: 10.1002/1097-0142(196203/04)15:2<364::aid-cncr2820150220>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Sunderman F. W., Jr, Mitchell J. M., Allpass P. R. Induction of testicular sarcomas in Fischer rats by intratesticular injection of nickel subsulfide. Cancer Res. 1978 Feb;38(2):268–276. [PubMed] [Google Scholar]
- Egan K. M., Seddon J. M., Glynn R. J., Gragoudas E. S., Albert D. M. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988 Jan-Feb;32(4):239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Font R. L., Moura R. A., Shetlar D. J., Martinez J. A., McPherson A. R. Combined hamartoma of sensory retina and retinal pigment epithelium. Retina. 1989;9(4):302–311. doi: 10.1097/00006982-198909040-00011. [DOI] [PubMed] [Google Scholar]
- Font R. L., Reynolds A. M., Jr, Zimmerman L. E. Diffuse malignant melanoma of the iris in the nevus of Ota. Arch Ophthalmol. 1967 Apr;77(4):513–518. doi: 10.1001/archopht.1967.00980020515014. [DOI] [PubMed] [Google Scholar]
- Font R. L., Spaulding A. G., Zimmerman LE DIFFUSE MALI a clinicopathologic report of 54 cases. Trans Am Acad Ophthalmol Otolaryngol. 1968 Nov-Dec;72(6):877–895. [PubMed] [Google Scholar]
- Frezzotti R., Guerra R., Dragoni G. P., Bonanni P. Malignant melanoma of the choroid in a case of naevus of ota. Br J Ophthalmol. 1968 Dec;52(12):922–924. doi: 10.1136/bjo.52.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst A., Haro R. T., Schlauder M. Experimental chemotherapy of nickel-induced fibrosarcomas. Oncology. 1972;26(5):422–426. doi: 10.1159/000224694. [DOI] [PubMed] [Google Scholar]
- GILMAN J. P. Metal carcinogenesis. II. A study on the carcinogenic activity of cobalt, copper, iron, and nickel compounds. Cancer Res. 1962 Feb;22:158–162. [PubMed] [Google Scholar]
- Gallie B. L., Albert D. M., Wong J. J., Buyukmihci N., Pullafito C. A. Heterotransplantation of retinoblastoma into the athymic "nude" mouse. Invest Ophthalmol Vis Sci. 1977 Mar;16(3):256–259. [PubMed] [Google Scholar]
- Gamel J. W., McLean I. W., Foster W. D., Zimmerman L. E. Uveal melanomas: correlation of cytologic features with prognosis. Cancer. 1978 May;41(5):1897–1901. doi: 10.1002/1097-0142(197805)41:5<1897::aid-cncr2820410534>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Gamel J. W., McLean I. W., Greenberg R. A., Zimmerman L. E., Lichtenstein S. J. Computerized histologic assessment of malignant potential: a method for determining the prognosis of uveal melanomas. Hum Pathol. 1982 Oct;13(10):893–897. doi: 10.1016/s0046-8177(82)80048-8. [DOI] [PubMed] [Google Scholar]
- Garner A. Tumours of the retinal pigment epithelium. Br J Ophthalmol. 1970 Nov;54(11):715–723. doi: 10.1136/bjo.54.11.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause P. R., Lluria-Prevatt M., Keith W. N., Balmain A., Linardopolous S., Warneke J., Powell M. B. Chromosomal and genetic alterations of 7,12-dimethylbenz[a]anthracene-induced melanoma from TP-ras transgenic mice. Mol Carcinog. 1997 Sep;20(1):78–87. doi: 10.1002/(sici)1098-2744(199709)20:1<78::aid-mc9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gonder J. R., Shields J. A., Albert D. M. Malignant melanoma of the choroid associated with oculodermal melanocytosis. Ophthalmology. 1981 Apr;88(4):372–376. doi: 10.1016/s0161-6420(81)35023-4. [DOI] [PubMed] [Google Scholar]
- Grossniklaus H. E., Barron B. C., Wilson M. W. Murine model of anterior and posterior ocular melanoma. Curr Eye Res. 1995 May;14(5):399–404. doi: 10.3109/02713689508999938. [DOI] [PubMed] [Google Scholar]
- Grossniklaus H. E., Wilson M. W., Barron B. C., Lynn M. J. Anterior vs posterior intraocular melanoma. Metastatic differences in a murine model. Arch Ophthalmol. 1996 Sep;114(9):1116–1120. doi: 10.1001/archopht.1996.01100140318011. [DOI] [PubMed] [Google Scholar]
- Haas B. D., Jakobiec F. A., Iwamoto T., Cox M., Bernacki E. G., Pokorny K. L. Diffuse choroidal melanocytoma in a child. A lesion extending the spectrum of melanocytic hamartomas. Ophthalmology. 1986 Dec;93(12):1632–1638. doi: 10.1016/s0161-6420(86)33519-x. [DOI] [PubMed] [Google Scholar]
- Halasa A. Malignant melanoma in a case of bilateral nevus of Ota. Arch Ophthalmol. 1970 Aug;84(2):176–178. doi: 10.1001/archopht.1970.00990040178010. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Satyamoorthy K. Activated ras. Yet another player in melanoma? Am J Pathol. 1996 Sep;149(3):739–744. [PMC free article] [PubMed] [Google Scholar]
- Juarez C. P., Tso M. O. An ultrastructural study of melanocytomas (magnocellular nevi) of the optic disk and uvea. Am J Ophthalmol. 1980 Jul;90(1):48–62. doi: 10.1016/s0002-9394(14)75076-7. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley T. A., Jr, King C. M. Malignant melanoma in melanosis oculi. Trans Am Acad Ophthalmol Otolaryngol. 1967 Jul-Aug;71(4):638–641. [PubMed] [Google Scholar]
- McLean E. B. Hamartoma of the retinal pigment epithelium. Am J Ophthalmol. 1976 Aug;82(2):227–231. doi: 10.1016/0002-9394(76)90423-2. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Zimmerman L. E., Evans R. M. Reappraisal of Callender's spindle a type of malignant melanoma of choroid and ciliary body. Am J Ophthalmol. 1978 Oct;86(4):557–564. doi: 10.1016/0002-9394(78)90307-0. [DOI] [PubMed] [Google Scholar]
- Naumann G., Yanoff M., Zimmerman L. E. Histogenesis of malignant melanomas of the uvea. I. Histopathologic characteristics of nevi of the choroid and ciliary body. Arch Ophthalmol. 1966 Dec;76(6):784–796. doi: 10.1001/archopht.1966.03850010786004. [DOI] [PubMed] [Google Scholar]
- Niederkorn J. Y. Suppressed cellular immunity in mice harboring intraocular melanomas. Invest Ophthalmol Vis Sci. 1984 Apr;25(4):447–454. [PubMed] [Google Scholar]
- Powell M. B., Hyman P., Bell O. D., Balmain A., Brown K., Alberts D., Bowden G. T. Hyperpigmentation and melanocytic hyperplasia in transgenic mice expressing the human T24 Ha-ras gene regulated by a mouse tyrosinase promoter. Mol Carcinog. 1995 Feb;12(2):82–90. doi: 10.1002/mc.2940120205. [DOI] [PubMed] [Google Scholar]
- Roy P. E., Schaeffer E. M. Nevus of Ota and choroidal melanoma. Surv Ophthalmol. 1967 Apr;12(2):130–140. [PubMed] [Google Scholar]
- Ryll D. L., Campbell R. J., Robertson D. M., Brubaker S. J. 7. Pseudometastatic lesions of the choroid. Ophthalmology. 1980 Nov;87(11):1181–1186. doi: 10.1016/s0161-6420(80)35107-5. [DOI] [PubMed] [Google Scholar]
- Sabates F. N., Yamashita T. Congenital melanosis oculi complicated by two independent malignant melanomas of the choroid. Arch Ophthalmol. 1967 Jun;77(6):801–803. doi: 10.1001/archopht.1967.00980020803018. [DOI] [PubMed] [Google Scholar]
- Schachat A. P., Shields J. A., Fine S. L., Sanborn G. E., Weingeist T. A., Valenzuela R. E., Brucker A. J. Combined hamartomas of the retina and retinal pigment epithelium. Ophthalmology. 1984 Dec;91(12):1609–1615. doi: 10.1016/s0161-6420(84)34094-5. [DOI] [PubMed] [Google Scholar]
- Schena M., Shalon D., Heller R., Chai A., Brown P. O., Davis R. W. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelenfreund M. H., Freilich D. B. Ocular melanocytosis and malignant melanoma. N Y State J Med. 1979 May;79(6):916–917. [PubMed] [Google Scholar]
- Streeten B. W. Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol. 1969 Mar;81(3):383–394. doi: 10.1001/archopht.1969.00990010385017. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Yamamoto H., Takeuchi S., Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990 Feb;108(2):223–227. doi: 10.1242/dev.108.2.223. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Albert D. M. Pathological condition of the retinal pigment epithelium. Neoplasms and nodular non-neoplastic lesions. Arch Ophthalmol. 1972 Jul;88(1):27–38. doi: 10.1001/archopht.1972.01000030029007. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Malignant melanoma of the choroid in the nevus of Ota. Ophthalmologica. 1969;159(1):1–10. doi: 10.1159/000305882. [DOI] [PubMed] [Google Scholar]
- Yanoff M., Zimmerman L. E. Histogenesis of malignant melanomas of the uvea. 3. The relationship of congenital ocular melanocytosis and neurofibromatosis in uveal melanomas. Arch Ophthalmol. 1967 Mar;77(3):331–336. doi: 10.1001/archopht.1967.00980020333007. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN L. E. MELANOCYTES, MELANOCYTIC NEVI, AND MELANOCYTOMAS. Invest Ophthalmol. 1965 Feb;4:11–41. [PubMed] [Google Scholar]