Abstract
AIM—To study the behaviour of residual lens epithelial cells after capsulorhexis and expression of material from the isolated lens. METHODS—Human and bovine lens capsules were isolated, sterile non-toxic silicone rings inserted, and the preparations placed in organ culture for up to 12 weeks. Cell coverage of the posterior lens capsule was recorded and the capsules were examined, both pre- and post-coverage, for (a) proliferative activity and (b) cytoskeletal components. RESULTS—After a lag period outgrowth was observed across the posterior capsule. The rate of cell coverage was dependent upon both species and the presence or absence of serum. The proliferative activity of the cells was greatest at or near the leading edge and decreased once covered. Wrinkles became visible in the posterior capsule during the late stages of pre-coverage and increased in both number and complexity. All cells on both the human and bovine posterior capsules contained F-actin and vimentin and the majority were immunolabelled for α-smooth muscle actin (α-SMA). CONCLUSIONS—This model exhibits many of the in vivo characteristics of the lens capsule after extracapsular surgery and may prove useful in further elucidating the cellular mechanisms of posterior capsule opacification. Keywords: lens epithelial cells; posterior capsule opacification; capsulorrhexis
Full Text
The Full Text of this article is available as a PDF (203.5 KB).
Figure 1 .
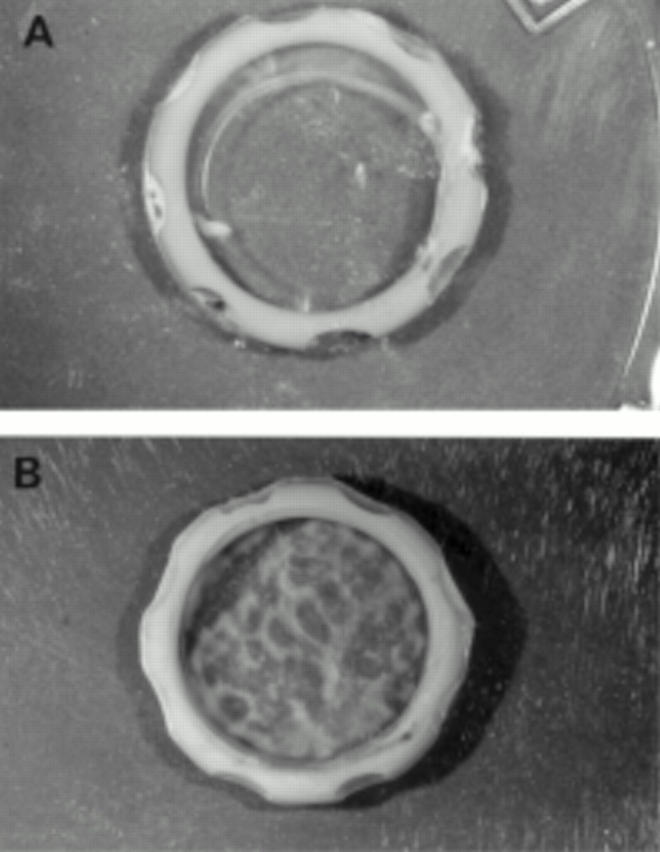
Bovine lens capsule with a silicone ring inserted. Immediately after insertion the capsule was clear (A) but after 8 weeks in serum containing culture opacification was evident (B). Magnification ×2.5.
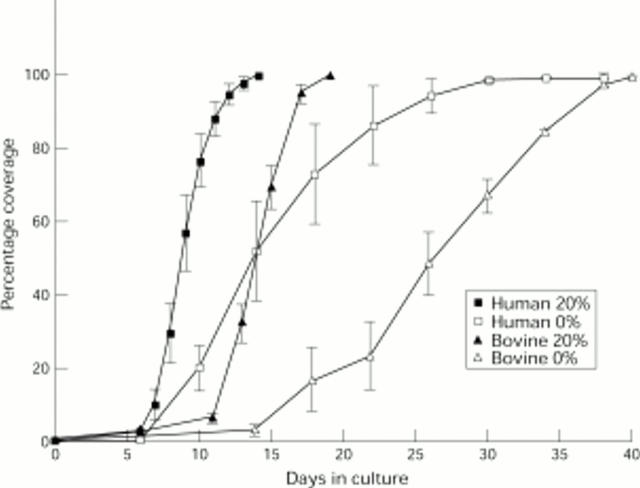
Figure 2 .
Graph showing the rate of coverage of bovine and human posterior capsules. The bovine data represent an average of results obtained for 18 capsules cultured in 20% FCS and three capsules in 0% FCS. The human data represent the average results obtained for 12 pairs of capsules, one of each pair in serum containing medium and the other in serum free medium. Vertical bars represent SEM.
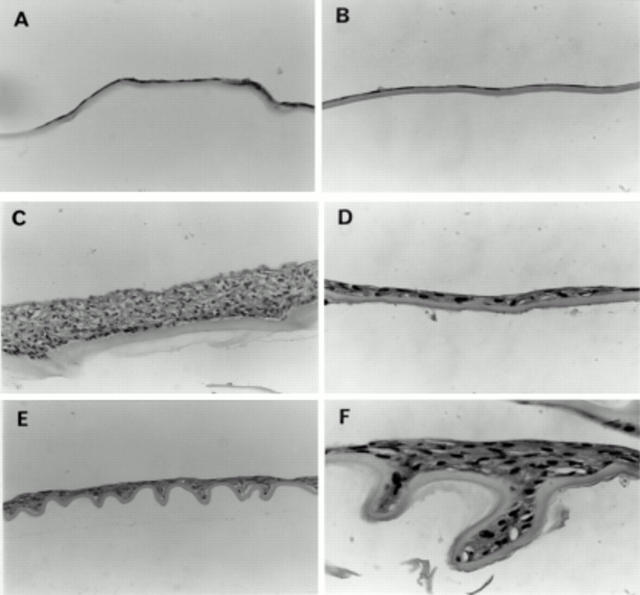
Figure 3 .
Histological sections of bovine (A, C, E) and human (B, D, F) posterior capsules at the different times of coverage; initial coverage as a monolayer (A, B), formation of multilayers (C, D), and wrinkling of the posterior capsule (E, F). Capsules were maintained in serum containing medium. Sections were stained with haematoxylin and eosin. Original magnification A, C, and E ×143; B, D, and F ×570.
Figure 4 .
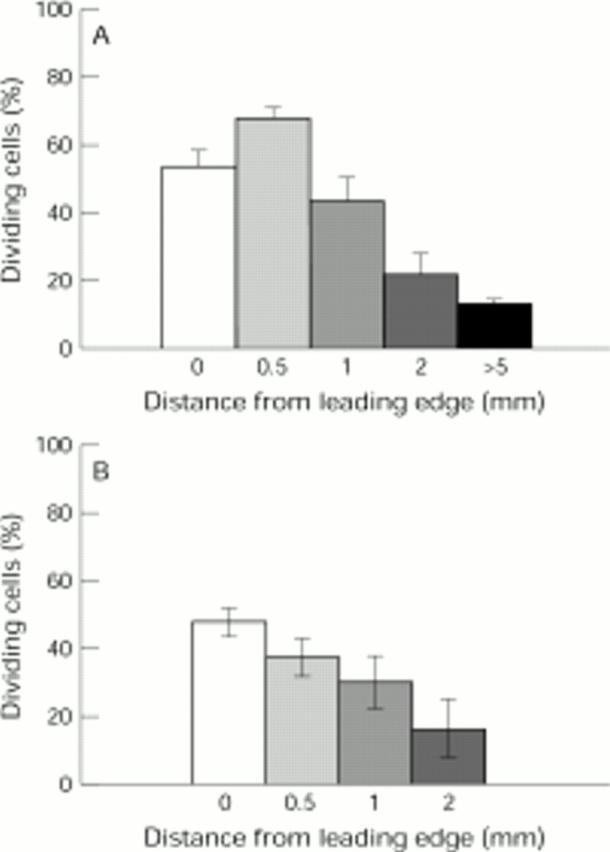
Proliferative activity of cells in the different regions of the bovine (A) and human (B) posterior capsule. The data represent the average of results obtained from six bovine and six human capsules in serum containing medium at approximately 50% coverage. Recordings were taken from the leading edge in three different regions of each capsule. Vertical bars represent SEM.
Figure 5 .
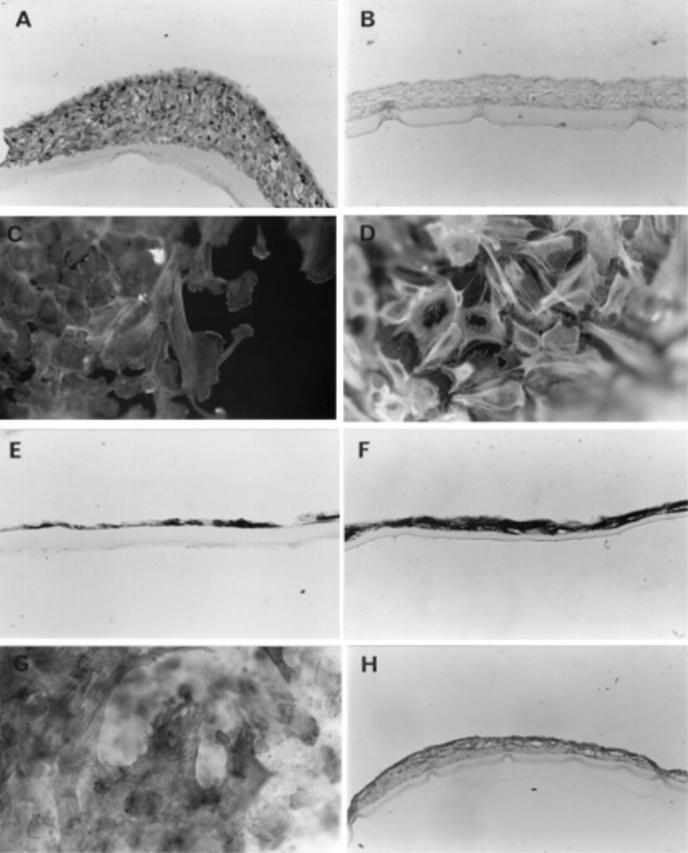
Localisation of vimentin (A), cytokeratin (B), F-actin (C, D), and α-SMA (E-H) in cells on the bovine (A, B, C, G, H) and human (D, E, F) posterior capsules at varying stages of coverage. All cells contained vimentin (A) but cytokeratin was absent (B). All cells stained for F-actin; microfilaments were polarised along the length of the cells at the leading edge (C) and cells away from the leading edge had a perinuclear basket of F-actin (D). α-SMA was randomly distributed in the pre-covered capsules (E) and found throughout the multilayers of the human post-covered capsules (F). In the post-covered bovine capsules α-SMA was present in all fibroblast-like cells (G) and throughout the multilayers although occasionally localised predominantly to the surface layers of the posterior capsule (H). Capsules were maintained in serum containing medium. Original magnification of A, B, D, H ×143, C ×160, E, F × 570, G ×64.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple D. J., Solomon K. D., Tetz M. R., Assia E. I., Holland E. Y., Legler U. F., Tsai J. C., Castaneda V. E., Hoggatt J. P., Kostick A. M. Posterior capsule opacification. Surv Ophthalmol. 1992 Sep-Oct;37(2):73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Behar-Cohen F. F., David T., D'Hermies F., Pouliquen Y. M., Buechler Y., Nova M. P., Houston L. L., Courtois Y. In vivo inhibition of lens regrowth by fibroblast growth factor 2-saporin. Invest Ophthalmol Vis Sci. 1995 Nov;36(12):2434–2448. [PubMed] [Google Scholar]
- Courtney P. The National Cataract Surgery Survey: I. Method and descriptive features. Eye (Lond) 1992;6(Pt 5):487–492. doi: 10.1038/eye.1992.103. [DOI] [PubMed] [Google Scholar]
- Green W. R., McDonnell P. J. Opacification of the posterior capsule. Trans Ophthalmol Soc U K. 1985;104(Pt 7):727–739. [PubMed] [Google Scholar]
- Hales A. M., Schulz M. W., Chamberlain C. G., McAvoy J. W. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994 Dec;13(12):885–890. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara T., Sakanishi K., Yamada Y. Efficacy of equator rings in an experimental rabbit study. Arch Ophthalmol. 1995 Aug;113(8):1060–1065. doi: 10.1001/archopht.1995.01100080112038. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara T., Yamada Y. "Equator ring" for maintenance of the completely circular contour of the capsular bag equator after cataract removal. Ophthalmic Surg. 1991 Jun;22(6):358–359. [PubMed] [Google Scholar]
- Ibaraki N., Lin L. R., Reddy V. N. Effects of growth factors on proliferation and differentiation in human lens epithelial cells in early subculture. Invest Ophthalmol Vis Sci. 1995 Oct;36(11):2304–2312. [PubMed] [Google Scholar]
- Kappelhof J. P., Vrensen G. F. The pathology of after-cataract. A minireview. Acta Ophthalmol Suppl. 1992;(205):13–24. doi: 10.1111/j.1755-3768.1992.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Kappelhof J. P., Vrensen G. F., de Jong P. T., Pameyer J., Willekens B. An ultrastructural study of Elschnig's pearls in the pseudophakic eye. Am J Ophthalmol. 1986 Jan 15;101(1):58–69. doi: 10.1016/0002-9394(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Kurosaka D., Kato K., Nagamoto T., Negishi K. Growth factors influence contractility and alpha-smooth muscle actin expression in bovine lens epithelial cells. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1701–1708. [PubMed] [Google Scholar]
- Liu C. S., Wormstone I. M., Duncan G., Marcantonio J. M., Webb S. F., Davies P. D. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):906–914. [PubMed] [Google Scholar]
- Lovicu F. J., Chamberlain C. G., McAvoy J. W. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1459–1469. [PubMed] [Google Scholar]
- Lundgren B., Jonsson E., Rolfsen W. Secondary cataract. An in vivo model for studies on secondary cataract in rabbits. Acta Ophthalmol Suppl. 1992;(205):25–28. [PubMed] [Google Scholar]
- McDonnell P. J., Patel A., Green W. R. Comparison of intracapsular and extracapsular cataract surgery. Histopathologic study of eyes obtained postmortem. Ophthalmology. 1985 Sep;92(9):1208–1225. doi: 10.1016/s0161-6420(85)33875-7. [DOI] [PubMed] [Google Scholar]
- McDonnell P. J., Rowen S. L., Glaser B. M., Sato M. Posterior capsule opacification. An in vitro model. Arch Ophthalmol. 1985 Sep;103(9):1378–1381. doi: 10.1001/archopht.1985.01050090130047. [DOI] [PubMed] [Google Scholar]
- McDonnell P. J., Stark W. J., Green W. R. Posterior capsule opacification: a specular microscopic study. Ophthalmology. 1984 Jul;91(7):853–856. doi: 10.1016/s0161-6420(84)34226-9. [DOI] [PubMed] [Google Scholar]
- Nagamoto T., Bissen-Miyajima H. A ring to support the capsular bag after continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1994 Jul;20(4):417–420. doi: 10.1016/s0886-3350(13)80177-0. [DOI] [PubMed] [Google Scholar]
- Odrich M. G., Hall S. J., Worgul B. V., Trokel S. L., Rini F. J. Posterior capsule opacification: experimental analyses. Ophthalmic Res. 1985;17(2):75–84. doi: 10.1159/000265354. [DOI] [PubMed] [Google Scholar]
- Ohrloff C., Schalnus R., Rothe R., Spitznas M. Role of the posterior capsule in the aqueous-vitreous barrier in aphakic and pseudophakic eyes. J Cataract Refract Surg. 1990 Mar;16(2):198–201. doi: 10.1016/s0886-3350(13)80730-4. [DOI] [PubMed] [Google Scholar]
- Olivero D. K., Furcht L. T. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1993 Sep;34(10):2825–2834. [PubMed] [Google Scholar]
- Rakic J. M., Galand A., Vrensen G. F. Separation of fibres from the capsule enhances mitotic activity of human lens epithelium. Exp Eye Res. 1997 Jan;64(1):67–72. doi: 10.1006/exer.1996.0179. [DOI] [PubMed] [Google Scholar]
- Spierer A., Barequet I., Rosner M., Solomon A. S., Martinowitz U. Reattachment of extraocular muscles using fibrin glue in a rabbit model. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):543–546. [PubMed] [Google Scholar]
- Stark W. J., Sommer A., Smith R. E. Changing trends in intraocular lens implantation. Arch Ophthalmol. 1989 Oct;107(10):1441–1444. doi: 10.1001/archopht.1989.01070020515030. [DOI] [PubMed] [Google Scholar]
- Sveinsson O. The ultrastructure of Elschnig's pearls in a pseudophakic eye. Acta Ophthalmol (Copenh) 1993 Feb;71(1):95–98. doi: 10.1111/j.1755-3768.1993.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Wormstone I. M., Liu C. S., Rakic J. M., Marcantonio J. M., Vrensen G. F., Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):396–404. [PubMed] [Google Scholar]
- Wunderlich K., Knorr M. Einfluss des Platelet-derived-growth-Faktors PDGF auf replikative Vorgänge bei kultivierten bovinen Linsenepithelzellen (BLEZ). Ophthalmologe. 1994 Feb;91(1):98–102. [PubMed] [Google Scholar]