Abstract
BACKGROUND/AIMS—Toxoplasma retinochoroiditis (TR) is an important cause of blindness and visual morbidity, affecting young adults. It has been postulated that some of the retinal damage observed in TR is due to antiretinal autoimmune mechanisms. METHODS—Humoral antiretinal autoimmunity in TR was investigated by indirect immunofluorescence (IIF) on normal human cadaveric retina and by a human retinal S-antigen ELISA. 36 patients with TR were separated on clinical grounds into those with first recurrence of disease (n=18) or those with multiple recurrences (n=18). Patients were also segregated into those with active (n=28) or quiescent disease (n=8). Serum from 16 normal controls (six with positive toxoplasma serology and 10 without) with no evidence of eye disease and 12 patients with idiopathic retinal vasculitis (IRV) were also tested. RESULTS—Sera from 34 of the 36 patients (94%) with TR demonstrated photoreceptor layer reactivity by IIF contrasting with six of 16 normal controls (p= <0.001) and three of 12 IRV patients (p= <0.001). Titres of antiphotoreceptor antibody were also higher among TR patients than controls. Sera from 27 of the 36 TR patients, 10 of 16 normals, and nine of 12 retinal vasculitis patients possessed anti-human retinal S-antigen antibodies at a titre of 1:400 or more as assessed by ELISA (p= >0.05). Antiretinal autoantibody as detected by IIF did not run in parallel with S-antigen reactivity. CONCLUSIONS—The data indicate that the extent of antiretinal reactivity within TR is not accounted for by anti-S-antigen antibodies alone. This remarkably high prevalence of antiphotoreceptor antibody in TR as opposed to that found in either healthy or disease controls suggest that these antibodies may be co-pathogenic in toxoplasma retinochoroiditis. Keywords: retinochoroiditis; toxoplasma; immunofluorescence; autoimmunity
Full Text
The Full Text of this article is available as a PDF (128.2 KB).
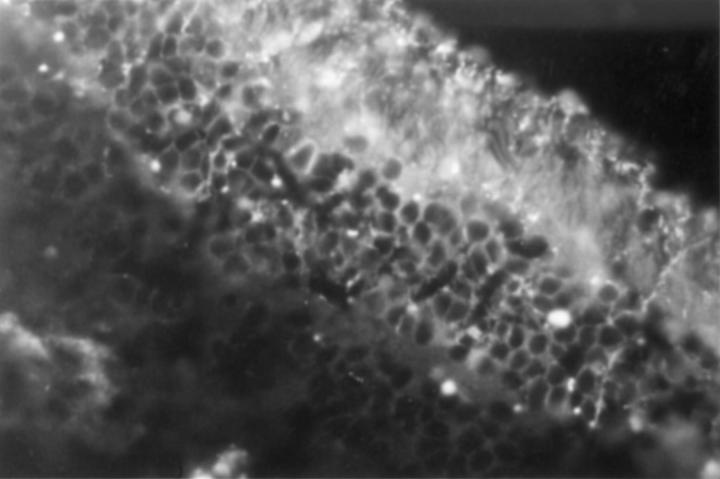
Figure 1 .
Indirect immunofluorescence of human retina (×40). Negative staining of all retinal layers. Note lipofuscin fragments of retinal pigment epithelia distributed across the section.
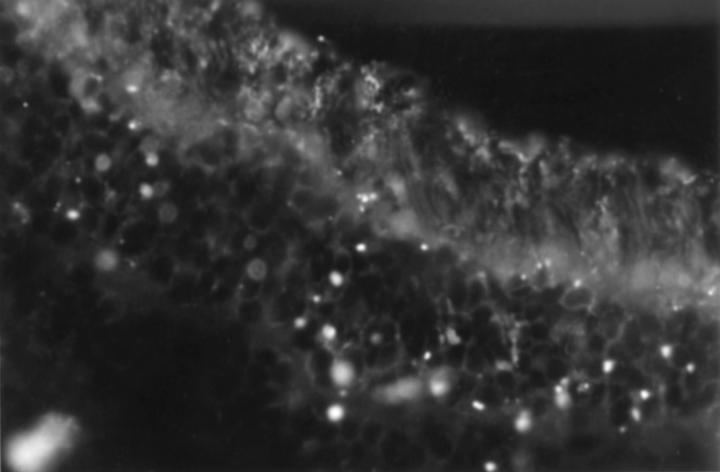
Figure 2 .
Indirect immunofluorescence of human retina (×40). Positive staining of photoreceptor cell cytoplasm and in both rods and cones by antibodies present in serum from a patient with TR (serum dilution 1:40).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams I. W., Gregerson D. S. Longitudinal study of serum antibody responses to retinal antigens in acute ocular toxoplasmosis. Am J Ophthalmol. 1982 Feb;93(2):224–231. doi: 10.1016/0002-9394(82)90418-4. [DOI] [PubMed] [Google Scholar]
- Ashton N. Ocular toxoplasmosis in wallabies (Macropus rufogriseus). Am J Ophthalmol. 1979 Sep;88(3 Pt 1):322–332. doi: 10.1016/0002-9394(79)90628-7. [DOI] [PubMed] [Google Scholar]
- Braun G., McKechnie N. M., Connor V., Gilbert C. E., Engelbrecht F., Whitworth J. A., Taylor D. W. Immunological crossreactivity between a cloned antigen of Onchocerca volvulus and a component of the retinal pigment epithelium. J Exp Med. 1991 Jul 1;174(1):169–177. doi: 10.1084/jem.174.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. C., Nussenblatt R. B., Kim M. K., Palestine A. G., Awadzi K., Ottesen E. A. Immunopathology of ocular onchocerciasis. 2. Anti-retinal autoantibodies in serum and ocular fluids. Ophthalmology. 1987 Apr;94(4):439–443. doi: 10.1016/s0161-6420(87)33452-9. [DOI] [PubMed] [Google Scholar]
- Dua H. S., Gomes J. A., Singh A., Eagle R. C., Jr, Donoso L. A., Laibson P. R. Fresh-frozen cucumber as a mount for conjunctival and corneal tissue in cryomicrotomy. Arch Ophthalmol. 1994 Sep;112(9):1139–1141. doi: 10.1001/archopht.1994.01090210023005. [DOI] [PubMed] [Google Scholar]
- Dutton G. N., Hay J. Toxoplasmic retinochoroiditis--current concepts in pathogenesis. Trans Ophthalmol Soc U K. 1983;103(Pt 5):503–507. [PubMed] [Google Scholar]
- Dutton G. N., McMenamin P. G., Hay J., Cameron S. The ultrastructural pathology of congenital murine toxoplasmic retinochoroiditis. Part II: The morphology of the inflammatory changes. Exp Eye Res. 1986 Oct;43(4):545–560. doi: 10.1016/s0014-4835(86)80022-7. [DOI] [PubMed] [Google Scholar]
- Dutton G. N. The causes of tissue damage in toxoplasmic retinochoroiditis. Trans Ophthalmol Soc U K. 1986;105(Pt 4):404–412. [PubMed] [Google Scholar]
- FRENKEL J. K., JACOBS L. Ocular toxoplasmosis; pathogenesis, diagnosis and treatment. AMA Arch Ophthalmol. 1958 Feb;59(2):260–279. [PubMed] [Google Scholar]
- FRENKEL J. K. Pathogenesis of toxoplasmosis with a consideration of cyst rupture in Besnoitia infection. Surv Ophthalmol. 1961 Dec;6:799–825. [PubMed] [Google Scholar]
- Friedmann C. T., Knox D. L. Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol. 1969 Apr;81(4):481–493. doi: 10.1001/archopht.1969.00990010483005. [DOI] [PubMed] [Google Scholar]
- Gilbert R. E., Stanford M. R., Jackson H., Holliman R. E., Sanders M. D. Incidence of acute symptomatic toxoplasma retinochoroiditis in south London according to country of birth. BMJ. 1995 Apr 22;310(6986):1037–1040. doi: 10.1136/bmj.310.6986.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner P. D., Silveira C., Kruszon-Moran D., Martins M. C., Burnier Júnior M., Silveira S., Camargo M. E., Nussenblatt R. B., Kaslow R. A., Belfort Júnior R. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992 Aug 15;114(2):136–144. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Abrahams I. W., Thirkill C. E. Serum antibody levels of uveitis patients to bovine retinal antigens. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):669–680. [PubMed] [Google Scholar]
- Hogan M. J., Moschini G. B., Zardi O. Effects of Toxoplasma gondii toxin on the rabbit eye. Am J Ophthalmol. 1971 Oct;72(4):733–742. doi: 10.1016/0002-9394(71)90011-0. [DOI] [PubMed] [Google Scholar]
- Kasp E., Banga J. P., Brown E. C., Wicking J. M., Suleyman S., Ellis B. A., Sanders M. D., Dumonde D. C. An improved method for the purification of retinal S-antigen using selective hydrophobic adsorption chromatography. J Immunol Methods. 1987 Jun 26;100(1-2):147–152. doi: 10.1016/0022-1759(87)90183-9. [DOI] [PubMed] [Google Scholar]
- Kean B. H. Clinical Toxoplasmosis--50 years. Trans R Soc Trop Med Hyg. 1972;66(4):549–571. doi: 10.1016/0035-9203(72)90300-8. [DOI] [PubMed] [Google Scholar]
- McKechnie N. M., Braun G., Connor V., Kläger S., Taylor D. W., Alexander R. A., Gilbert C. E. Immunologic cross-reactivity in the pathogenesis of ocular onchocerciasis. Invest Ophthalmol Vis Sci. 1993 Sep;34(10):2888–2902. [PubMed] [Google Scholar]
- Newman P. E., Ghosheh R., Tabbara K. F., O'Connor G. R., Stern W. The role of hypersensitivity reactions to toxoplasma antigens in experimental ocular toxoplasmosis in nonhuman primates. Am J Ophthalmol. 1982 Aug;94(2):159–164. doi: 10.1016/0002-9394(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Gery I., Ballintine E. J., Wacker W. B. Cellular immune responsiveness of uveitis patients to retinal S-antigen. Am J Ophthalmol. 1980 Feb;89(2):173–179. doi: 10.1016/0002-9394(80)90108-7. [DOI] [PubMed] [Google Scholar]
- Perkins E. S. Ocular toxoplasmosis. Br J Ophthalmol. 1973 Jan;57(1):1–17. doi: 10.1136/bjo.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. A., Font R. L. Toxoplasmic retinochoroiditis: electron-microscopic and immunofluorescence studies of formalin-fixed tissue. Arch Ophthalmol. 1977 Feb;95(2):273–277. doi: 10.1001/archopht.1977.04450020074012. [DOI] [PubMed] [Google Scholar]
- Rothova A. Ocular involvement in toxoplasmosis. Br J Ophthalmol. 1993 Jun;77(6):371–377. doi: 10.1136/bjo.77.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lelij A., Doekes G., Hwan B. S., Vetter J. C., Rietveld E., Stilma J. S., Kijlstra A. Humoral autoimmune response against S-antigen and IRBP in ocular onchocerciasis. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1374–1380. [PubMed] [Google Scholar]
- Wyler D. J., Blackman H. J., Lunde M. N. Cellular hypersensitivity to toxoplasmal and retinal antigens in patients with toxoplasmal retinochoroiditis. Am J Trop Med Hyg. 1980 Nov;29(6):1181–1186. doi: 10.4269/ajtmh.1980.29.1181. [DOI] [PubMed] [Google Scholar]
- Yeo J. H., Jakobiec F. A., Iwamoto T., Richard G., Kreissig I. Opportunistic toxoplasmic retinochoroiditis following chemotherapy for systemic lymphoma. A light and electron microscopic study. Ophthalmology. 1983 Aug;90(8):885–898. doi: 10.1016/s0161-6420(83)80012-8. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M. O. Experimental Toxoplasma retinitis: a light and electron microscopical study. Arch Pathol Lab Med. 1976 Sep;100(9):487–490. [PubMed] [Google Scholar]