Abstract
AIMS—To establish a simple model of conjunctival wound healing in the mouse eye. METHODS—4 week old BALB/c mouse eyes were studied over a 14 day period. Surgical procedure under general anaesthesia involved a blunt dissection of the conjunctiva performed by injection of 25 µl of PBS via a 27 gauge needle into one eye, while the contralateral eye was used as control. Mice were assessed clinically and sacrificed at 1, 2, 3, 7, and 14 days after surgery. Enucleated eyes were prepared for histological analysis. Development of scar tissue was studied with haematoxylin and eosin, oxidation aldehyde fuchsin, and van Gieson stains, with assessment of cellularity, extracellular matrix formation, and wound characterisation. RESULTS—Histological analysis revealed a marked and characteristic healing response initiated by a predominantly granulocytic inflammatory reaction at day 1 with peak fibroblast activity 3 days after surgery. Oxytalan fibres and newly laid collagen fibres were detected early in the subconjunctival wound area and up to 7 days after surgery. Remodelling and complete organisation of scar tissue was evident by day 14. CONCLUSION—A single subconjunctival injection in the mouse eye results in a marked and consistent healing response. This represents a simple, inexpensive, and reliable animal model of conjunctival scarring. The mouse is a biologically well characterised animal model and allows the use of a wide variety of molecular tools. There are potentially significant clinical applications, in particular in investigating the effects of modulating agents such as antimetabolites, growth factors, and their antagonists on conjunctival scarring. Keywords: animal model; conjunctival scarring; mouse; wound healing
Full Text
The Full Text of this article is available as a PDF (197.7 KB).
Figure 1 .
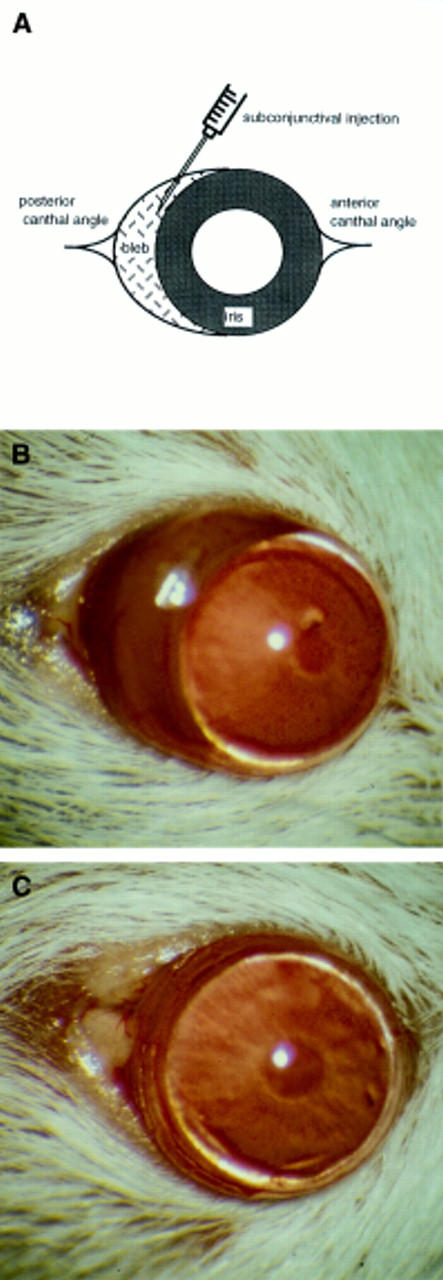
Subconjunctival injections of 25 µl of PBS were given 0.5 mm behind the limbus and in close proximity to the posterior canthal angle (A) of each mouse eye. A subconjunctival bleb was visible in all eyes following the subconjunctival injection and was macroscopically apparent until 24 hours (B). At 14 days, the macroscopic appearance of the eye that had received a subconjunctival injection was similar to that of the control eye (C).
Figure 2 .
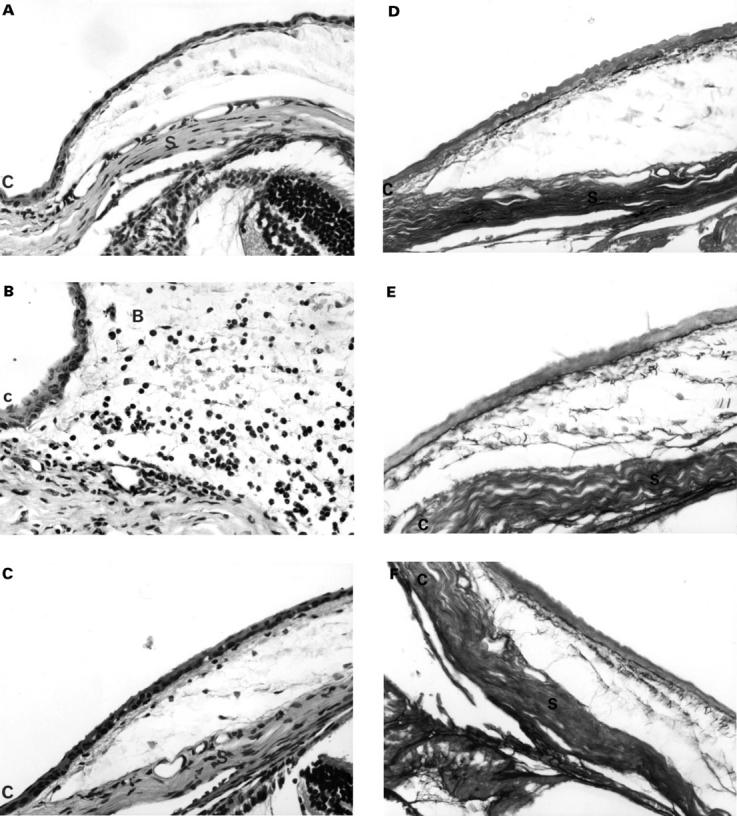
Histological evaluation of the cellular effects of the subconjunctival injection showed a typical wound healing response. Compared with the control eye (A), an initial influx of granulocytes (neutrophil polymorphonuclear leucocytes) was seen in association with considerable oedema of the subconjunctival tissue at day 1 in injected eyes (B). However, by day 14 (C) granulocytic and mononuclear activity had returned to control level. There was an increase in fibroblast number in the first several days after the injection, reaching a peak at day 3. Thereafter, fibroblast activity was found to decrease but did not ever return to the control eye activity level (haematoxylin and eosin stained 5 µm thick sections, × 80 magnification. Cornea=C, bleb=B, and sclera=S). Histological demonstration of extracellular matrix deposition in the subconjunctival bleb area using oxidation aldehyde-fuchsin stains. Early deposition of oxytalan and related fibres was demonstrated by the presence of dark, fibrillar fibres. Compared with control (D), increased deposition in injected eyes was seen at day 3 (E). An increased in amount and the degree of organisation of elastic related fibres was seen in injected eyes at day 14 compared with control (F). (Aldehyde-fuchsin stained 5 µm thick sections, ×80 magnification).
Figure 3 .
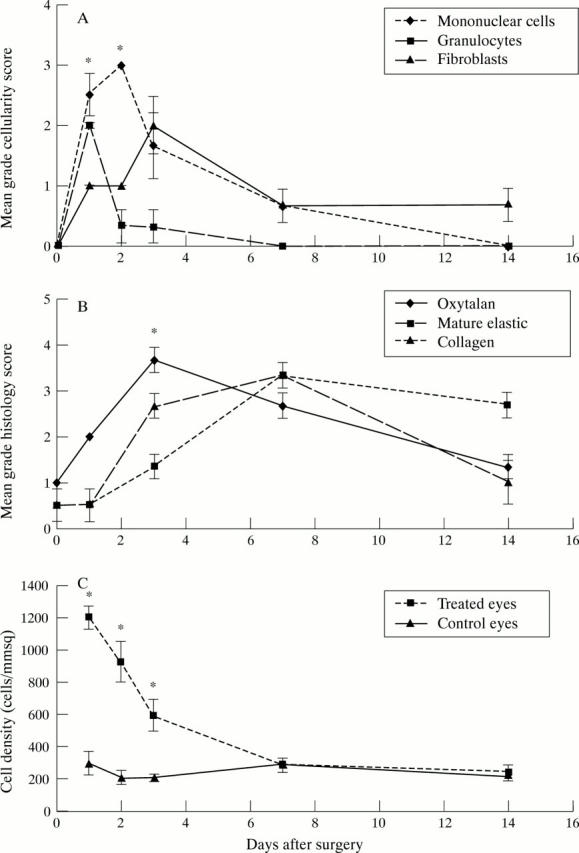
(A) Graph showing the cellular profile of mononuclear cells, granulocytes, and fibroblast activity in the bleb area over time, as graded by a masked observer (IAC). The consecutive peaks of these cell types demonstrate the classic wound healing response. (B) Graph showing the histochemical staining characteristics and the conjunctival scarring response. Newly laid extracellular matrix is demonstrated by the early deposition of oxytalan fibres, which later undergo maturation into elastic fibres. Collagen deposition occurs in association with wound remodelling, organisation, and contraction. (C) Graph showing the cellular density in injected eyes compared with their contralateral control eyes. A fourfold increase in cellular density is seen at day 1. (All graphs show SEM error bars, with * indicating statistical significant differences between groups p<0.05.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addicks E. M., Quigley H. A., Green W. R., Robin A. L. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983 May;101(5):795–798. doi: 10.1001/archopht.1983.01040010795021. [DOI] [PubMed] [Google Scholar]
- Alexander R. A., Hiscott P., McGalliard J., Grierson I. Oxytalan fibres in proliferative vitreoretinopathy. Ger J Ophthalmol. 1992;1(6):382–387. [PubMed] [Google Scholar]
- Bergstrom T. J., Wilkinson W. S., Skuta G. L., Watnick R. L., Elner V. M. The effects of subconjunctival mitomycin-C on glaucoma filtration surgery in rabbits. Arch Ophthalmol. 1991 Dec;109(12):1725–1730. doi: 10.1001/archopht.1991.01080120109038. [DOI] [PubMed] [Google Scholar]
- Costa V. P., Spaeth G. L., Eiferman R. A., Orengo-Nania S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993 Mar;24(3):152–170. [PubMed] [Google Scholar]
- Desjardins D. C., Parrish R. K., 2nd, Folberg R., Nevarez J., Heuer D. K., Gressel M. G. Wound healing after filtering surgery in owl monkeys. Arch Ophthalmol. 1986 Dec;104(12):1835–1839. doi: 10.1001/archopht.1986.01050240109050. [DOI] [PubMed] [Google Scholar]
- Doyle J. W., Sherwood M. B., Khaw P. T., McGrory S., Smith M. F. Intraoperative 5-fluorouracil for filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3313–3319. [PubMed] [Google Scholar]
- Gressel M. G., Parrish R. K., 2nd, Folberg R. 5-fluorouracil and glaucoma filtering surgery: I. An animal model. Ophthalmology. 1984 Apr;91(4):378–383. doi: 10.1016/s0161-6420(84)34277-4. [DOI] [PubMed] [Google Scholar]
- Herschler J., Claflin A. J., Fiorentino G. The effect of aqueous humor on the growth of subconjunctival fibroblasts in tissue culture and its implications for glaucoma surgery. Am J Ophthalmol. 1980 Feb;89(2):245–249. doi: 10.1016/0002-9394(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Hitchings R. A., Grierson I. Clinico pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc U K. 1983;103(Pt 1):84–88. [PubMed] [Google Scholar]
- Joseph J. P., Grierson I., Hitchings R. A. Chemotactic activity of aqueous humor. A cause of failure of trabeculectomies? Arch Ophthalmol. 1989 Jan;107(1):69–74. doi: 10.1001/archopht.1989.01070010071030. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Occleston N. L., Schultz G., Grierson I., Sherwood M. B., Larkin G. Activation and suppression of fibroblast function. Eye (Lond) 1994;8(Pt 2):188–195. doi: 10.1038/eye.1994.44. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Sherwood M. B., MacKay S. L., Rossi M. J., Schultz G. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon's capsule fibroblasts. Arch Ophthalmol. 1992 Aug;110(8):1150–1154. doi: 10.1001/archopht.1992.01080200130040. [DOI] [PubMed] [Google Scholar]
- Kirsner R. S., Eaglstein W. H. The wound healing process. Dermatol Clin. 1993 Oct;11(4):629–640. [PubMed] [Google Scholar]
- Mehra K. S., Dube B., Dube R. K. Fibrinolytic activity in blood and aqueous humour in glaucoma. Indian J Ophthalmol. 1983;31 (Suppl):827–829. [PubMed] [Google Scholar]
- Miller A. D. Retrovirus packaging cells. Hum Gene Ther. 1990 Spring;1(1):5–14. doi: 10.1089/hum.1990.1.1-5. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Grierson I., Unger W. I., Hitchings R. A. Wound healing in an animal model of glaucoma fistulizing surgery in the rabbit. Ophthalmic Surg. 1989 May;20(5):350–357. [PubMed] [Google Scholar]
- Miller M. H., Joseph N. H., Ennis K. W., Grierson I., Hitchings R. A. An animal model of filtration surgery. Trans Ophthalmol Soc U K. 1985;104(Pt 8):893–897. [PubMed] [Google Scholar]
- Radius R. L., Herschler J., Claflin A., Fiorentino G. Aqueous humor changes after experimental filtering surgery. Am J Ophthalmol. 1980 Feb;89(2):250–254. doi: 10.1016/0002-9394(80)90119-1. [DOI] [PubMed] [Google Scholar]
- SNELL A. C., Jr Wound healing of the iris in the presence of autologously transplanted granulation tissue. Am J Ophthalmol. 1956 Mar;41(3):499–505. [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995 Mar;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994 May;107(Pt 5):1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Sheridan C. M., Unger W. G., Ayliffe W., Alam Y., Goldsmith J., O'Donoghue E., McLeod D. Macrophages during fibrosis following scleral fistulising surgery in a rat model. Curr Eye Res. 1996 May;15(5):559–568. doi: 10.3109/02713689609000767. [DOI] [PubMed] [Google Scholar]


