Abstract
BACKGROUND—Amblyopia results in deficits in a number of visual functions in both the amblyopic and dominant eye. The present work describes oscillatory movement displacement thresholds (OMDT) in childhood amblyopia. METHODS—The OMDT from the dominant and amblyopic eyes of 50 orthoptic patients (aged 74 (SD 16) months) were compared with those from a group of 24 controls (79 (21) months). OMDT were measured using a forced choice staircase procedure. Subjects were asked to identify which of the computer controlled monitors displayed the oscillating stimulus. Visual acuity and stereoscopic responses were noted from clinical records. RESULTS—Amblyopic children demonstrating stereopsis showed no significant OMDT deficit in the amblyopic eye. Those children having no stereopsis had elevated OMDT in the amblyopic eye (p<0.05). Results suggest that the dominant eye of children with amblyopia may also have a pattern of visual development which is anomalous (difference in correlation coefficient with age; p <0.05). CONCLUSION—OMDT deficits demonstrated in some amblyopic eyes indicate that amblyopia is incompletely described by its "clinical" definition. Results suggest that the dominant eye in those with unilateral amblyopia may not be "normal". Keywords: amblyopia; children; vision; movement hyperacuity; stereopsis
Full Text
The Full Text of this article is available as a PDF (98.9 KB).
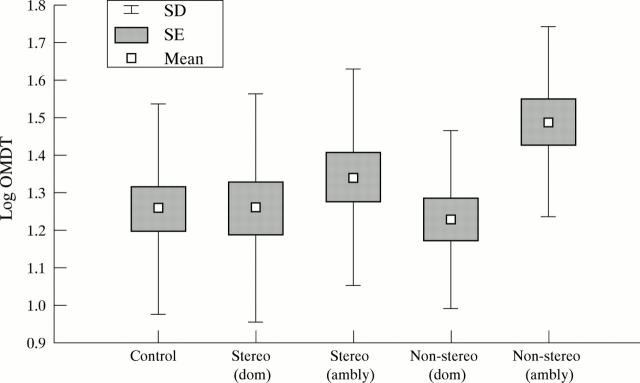
Figure 1 .
Mean log oscillatory movement displacement threshold (OMDT) in dominant and amblyopic eyes in stereopositive and stereonegative children compared with normal controls.
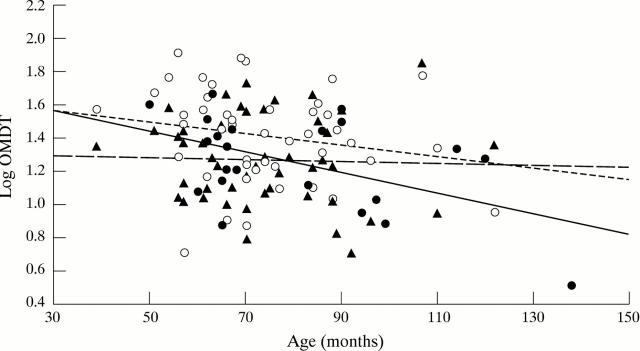
Figure 2 .
Log oscillatory movement displacement threshold (OMDT) shown as a function of age for amblyopic (∘, dominant (▴) and normal control eyes(•). Best fit lines are drawn for normal control (solid line) and amblyopic (dotted line) eyes, which show significant correlation with age. There is no significant correlation between OMDT and age in dominant eyes, although an indication of the best fit line is given (broken line).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson M., Sjöstrand J. Contrast sensitivity and acuity relationship in strabismic and anisometropic amblyopia. Br J Ophthalmol. 1988 Jan;72(1):44–49. doi: 10.1136/bjo.72.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. Early visual development: differential functioning of parvocellular and magnocellular pathways. Eye (Lond) 1992;6(Pt 2):129–135. doi: 10.1038/eye.1992.28. [DOI] [PubMed] [Google Scholar]
- Braddick O. J., Atkinson J. Some recent findings on the development of human binocularity: a review. Behav Brain Res. 1983 Oct;10(1):141–150. doi: 10.1016/0166-4328(83)90160-2. [DOI] [PubMed] [Google Scholar]
- Buckingham T., Watkins R., Bansal P., Bamford K. Hyperacuity thresholds for oscillatory movement are abnormal in strabismic and anisometropic amblyopes. Optom Vis Sci. 1991 May;68(5):351–356. doi: 10.1097/00006324-199105000-00005. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Fox K., Sato H., Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992 Jan;67(1):197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Daw N. W. Mechanisms of plasticity in the visual cortex. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994 Dec;35(13):4168–4179. [PubMed] [Google Scholar]
- Dobson V., Sebris S. L. Longitudinal study of acuity and stereopsis in infants with or at-risk for esotropia. Invest Ophthalmol Vis Sci. 1989 Jun;30(6):1146–1158. [PubMed] [Google Scholar]
- Epelbaum M., Milleret C., Buisseret P., Dufier J. L. The sensitive period for strabismic amblyopia in humans. Ophthalmology. 1993 Mar;100(3):323–327. doi: 10.1016/s0161-6420(13)32170-8. [DOI] [PubMed] [Google Scholar]
- Freeman R. D., Bradley A. Monocularly deprived humans: nondeprived eye has supernormal vernier acuity. J Neurophysiol. 1980 Jun;43(6):1645–1653. doi: 10.1152/jn.1980.43.6.1645. [DOI] [PubMed] [Google Scholar]
- Giaschi D. E., Regan D., Kraft S. P., Hong X. H. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Invest Ophthalmol Vis Sci. 1992 Jul;33(8):2483–2489. [PubMed] [Google Scholar]
- Hillis A. Amblyopia: prevalent, curable, neglected. Public Health Rev. 1986;14(3-4):213–235. [PubMed] [Google Scholar]
- Hiscox F., Strong N., Thompson J. R., Minshull C., Woodruff G. Occlusion for amblyopia: a comprehensive survey of outcome. Eye (Lond) 1992;6(Pt 3):300–304. doi: 10.1038/eye.1992.59. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Kandel G. L., Grattan P. E., Bedell H. E. Are the dominant eyes of amblyopes normal? Am J Optom Physiol Opt. 1980 Jan;57(1):1–6. doi: 10.1097/00006324-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Koskela P. U., Airaksinen P. J., Tuulonen A. The effect of jogging on visual field indices. Acta Ophthalmol (Copenh) 1990 Feb;68(1):91–93. doi: 10.1111/j.1755-3768.1990.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Leguire L. E., Rogers G. L., Bremer D. L. Amblyopia: the normal eye is not normal. J Pediatr Ophthalmol Strabismus. 1990 Jan-Feb;27(1):32–39. doi: 10.3928/0191-3913-19900101-10. [DOI] [PubMed] [Google Scholar]
- Leibowitz H. M., Krueger D. E., Maunder L. R., Milton R. C., Kini M. M., Kahn H. A., Nickerson R. J., Pool J., Colton T. L., Ganley J. P. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980 May-Jun;24(Suppl):335–610. [PubMed] [Google Scholar]
- Levartovsky S., Oliver M., Gottesman N., Shimshoni M. Factors affecting long term results of successfully treated amblyopia: initial visual acuity and type of amblyopia. Br J Ophthalmol. 1995 Mar;79(3):225–228. doi: 10.1136/bjo.79.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. Vernier acuity, crowding and amblyopia. Vision Res. 1985;25(7):979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. Differences in vernier discrimination for grating between strabismic and anisometropic amblyopes. Invest Ophthalmol Vis Sci. 1982 Sep;23(3):398–407. [PubMed] [Google Scholar]
- Lewis T. L., Maurer D., Tytla M. E., Bowering E. R., Brent H. P. Vision in the "good" eye of children treated for unilateral congenital cataract. Ophthalmology. 1992 Jul;99(7):1013–1017. doi: 10.1016/s0161-6420(92)31857-3. [DOI] [PubMed] [Google Scholar]
- Rutstein R. P., Fuhr P. S. Efficacy and stability of amblyopia therapy. Optom Vis Sci. 1992 Oct;69(10):747–754. doi: 10.1097/00006324-199210000-00001. [DOI] [PubMed] [Google Scholar]
- Stewart-Brown S. L., Haslum M. N., Howlett B. Preschool vision screening: a service in need of rationalisation. Arch Dis Child. 1988 Apr;63(4):356–359. doi: 10.1136/adc.63.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C., Gallant J. L. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994 Jul;13(1):1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Wali N., Leguire L. E., Rogers G. L., Bremer D. L. CSF interocular interactions in childhood ambylopia. Optom Vis Sci. 1991 Feb;68(2):81–87. doi: 10.1097/00006324-199102000-00001. [DOI] [PubMed] [Google Scholar]
- Watkins R., Buckingham T. Visual dysfunction in type II diabetic patients revealed by a hyperacuity test. Acta Ophthalmol (Copenh) 1992 Oct;70(5):659–664. doi: 10.1111/j.1755-3768.1992.tb02149.x. [DOI] [PubMed] [Google Scholar]
- Wick B., Wingard M., Cotter S., Scheiman M. Anisometropic amblyopia: is the patient ever too old to treat? Optom Vis Sci. 1992 Nov;69(11):866–878. doi: 10.1097/00006324-199211000-00006. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. Adult amblyopia reversed by contralateral cataract formation. J Pediatr Ophthalmol Strabismus. 1992 Mar-Apr;29(2):100–102. doi: 10.3928/0191-3913-19920301-09. [DOI] [PubMed] [Google Scholar]