Abstract
Cyclooxygenase-2 (COX-2)-catalyzed synthesis of prostaglandin E2 (PGE2) plays a key role in inflammation and its associated diseases, such as cancer and vascular heart disease. Here we report that γ-tocopherol (γT) reduced PGE2 synthesis in both lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and IL-1β-treated A549 human epithelial cells with an apparent IC50 of 7.5 and 4 μM, respectively. The major metabolite of dietary γT, 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman (γ-CEHC), also exhibited an inhibitory effect, with an IC50 of ≈30 μM in these cells. In contrast, α-tocopherol at 50 μM slightly reduced (25%) PGE2 formation in macrophages, but had no effect in epithelial cells. The inhibitory effects of γT and γ-CEHC stemmed from their inhibition of COX-2 activity, rather than affecting protein expression or substrate availability, and appeared to be independent of antioxidant activity. γ-CEHC also inhibited PGE2 synthesis when exposed for 1 h to COX-2-preinduced cells followed by the addition of arachidonic acid (AA), whereas under similar conditions, γT required an 8- to 24-h incubation period to cause the inhibition. The inhibitory potency of γT and γ-CEHC was diminished by an increase in AA concentration, suggesting that they might compete with AA at the active site of COX-2. We also observed a moderate reduction of nitrite accumulation and suppression of inducible nitric oxide synthase expression by γT in lipopolysaccharide-treated macrophages. These findings indicate that γT and its major metabolite possess anti-inflammatory activity and that γT at physiological concentrations may be important in human disease prevention.
Inflammatory diseases affect millions of people in the world, and chronic inflammation is one of the major contributors to the development of cancer as well as neurodegenerative and cardiovascular diseases (1, 2). Antioxidant vitamins, which defend against oxidants such as those produced during inflammation, are believed to play an important role in public health and human disease prevention (2). Among these vitamins, α-tocopherol (αT), the predominant form of vitamin E in many tissues and the exclusive component in most vitamin E supplements, has been extensively studied both in vitro and in vivo (3, 4). In contrast, γ-tocopherol (γT), while constituting 70–80% of vitamin E in U.S. diets (5), was mostly ignored in the past because of its relatively low animal plasma and tissue concentrations (6, 7), which result in a poor bioactivity as defined by the rat fetal resorption assay (8).
However, emerging evidence indicates that γT may be important in the defense against degenerative diseases. Some epidemiological studies suggest that high intakes or high plasma levels of αT predict low incidence of heart disease (9, 10), whereas others fail to observe the inverse correlation (11, 12) or protective effects from αT supplementation (13, 14). Instead, several independent investigations (11, 12, 15) demonstrate that plasma concentrations of γT, but not αT, are inversely correlated to the incidence of coronary heart diseases. Cooney et al. (16) and our laboratory (17) have shown that γT is superior to αT in trapping reactive nitrogen oxide species (NOx), mutagenic electrophiles generated during inflammation. Because of the nonsubstituted 5-position, γT is a better nucleophile and detoxifies NOx by forming a stable adduct, 5-nitro-γT (17–19). In an in vitro system, γT, compared with αT, exhibits stronger inhibition of lipid peroxidation induced by peroxynitrite (17). Dietary γT is primarily metabolized to 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman (γ-CEHC), a water-soluble compound found in human urine and possessing natriuretic activity (20, 21).
We have recently observed that γT supplementation leads to the inhibition of protein nitration and ascorbate oxidation, and spares vitamin C in rats with zymosan-induced peritonitis (Q.J., J. Lykkesfeldt, E. T. Shigeno, B.N.A., M. K. Shigenaga, and S. Christen, unpublished data). In addition to its reactivity toward NOx, it appears that γT plays a role in defending against inflammation-related damage. In the present study, we investigated the effects of γT on the inflammatory response in macrophages and human epithelial cells. We found that γT inhibited the generation of prostaglandin E2 (PGE2), an important mediator synthesized via the cyclooxygenase-2 (COX-2)-catalyzed oxidation of arachidonic acid (AA) during inflammation. Our results show that both γT and its major hydrophilic metabolite, γ-CEHC, at physiological concentrations are effective in inhibiting COX-2 activity in intact cells, whereas αT is much less effective.
Materials and Methods
Materials.
αT (99%) and γT (95–97%) were purchased from Acros Organics (Summerville, NJ) or Fluka. γ-CEHC (≥98%) and lipid hydroperoxide-free AA were from Cayman Chemicals (Ann Arbor, MI). 2′,7′-Dichlorofluorescin (DCFH) was from Molecular Probes. Tissue culture reagents were all from GIBCO/BRL. Bacterial lipopolysaccharide (LPS; B E. coli 055:B5) was from Difco. IL-1β and all other chemicals were from Sigma.
Cell Culture.
Murine RAW264.7 macrophages were routinely cultured in DMEM containing 10% FBS. Human epithelial cancer cells (A549) were obtained from American Type Culture Collection and cultured in F12K medium supplemented with 10% FBS.
Cellular Uptake of αT and γT.
Cells were incubated in DMEM containing 0.5% FBS supplemented with 10 μM αT or γT for 14 h. After harvested by scraping, cells were washed twice with Hanks' balanced salt solution. αT and γT were measured by HPLC by using electrochemical detection. The amounts of αT and γT in macrophages were 0.87 ± 0.12 and 1.05 ± 0.1 nmol/106 cells, and in epithelial cells were 0.90 ± 0.1 and 1.25 ± 0.2 nmol/106 cells, respectively.
PGE2 Generation During Chronic LPS and IL-1β Treatment.
RAW264.7 cells (5 × 105 per well) were allowed to attach overnight in a 24-well plate. Confluent cells were incubated with DMEM containing 0.5% FBS with ethanol (control) or tocopherols for the indicated time and then 0.1 μg/ml LPS was introduced for 14 h. Similarly, confluent A549 cells were incubated with DMEM containing 0.5% FBS supplemented with ethanol or various tocopherols for 8 h and then treated with 10 ng/ml IL-1β for 24 h, at which time the medium was collected and PGE2 generation was measured.
COX-2 Activity in Intact Cells.
A549 cells were pretreated with 10 ng/ml IL-1β for 24 h, then incubated with fresh medium containing tocopherols or ethanol for the indicated time, and finally incubated with exogenous AA for 10 min. PGE2 release was measured as an index of COX-2 activity.
Nitrite Measurement and DCFH Oxidation.
Formation of NOx was determined by nitrite accumulation in LPS-activated macrophages by using the Griess assay (22). The generation of total reactive oxygen species was evaluated by the oxidation of nonfluorescent DCFH to the highly fluorescent 2′,7′-dichlorofluorescein, as monitored by the increase in fluorescence intensity at 520 nm with excitation at 485 nm (23), by using a Cytofluor 2350 fluorescent measurement system (Millipore).
PGE2, Prostaglandin D2 (PGD2), and Isoprostane.
PGE2 and 8-isoprostane synthesis were determined by an enzymatic immunoassay (Cayman Chemicals), in which highly specific mAbs were used. PGD2 was first converted to PGD2 methoxime by reacting with methoxylamine and the derivative was determined by an immunoassay from Cayman Chemicals.
Western Blot.
Cells were pretreated with tocopherols for at least 8 h as described above. Optimal protein induction of COX-2 and inducible nitric oxide synthase (iNOS) in RAW264.7 cells was observed at 8 and 14 h, respectively, after LPS treatment. Harvested cells were broken by sonication in Hepes buffer with protease inhibitor mixture (Sigma), and protein concentrations were measured by the bicinchoninic acid (BCA) assay (Pierce). SDS/PAGE was performed on a precast 10% gel for COX-2 and 7.5% gel (Bio-Rad) for iNOS. Resolved proteins were transferred onto poly(vinylidene difluoride) membrane (Millipore) by either semidry or wet transfer. After blotting, proteins were probed by primary antibodies and secondary antibody (Santa Cruz Biotechnology) that is conjugated to horseradish peroxidase. Finally, membrane was exposed to chemiluminescent reagent (NEN, Life Science Products). Proteins were visualized on a Kodak film by using a M35A X-OMAT processor (Kodak) and quantified by ALPHAEASE 4.0 (Alpha Innotech, San Leandro, CA) or IMAGEQUANT 1.2 (Molecular Dynamics).
Northern Blot.
RAW264.7 macrophages were pretreated with tocopherols for 8–14 h and then stimulated with LPS for 8 h. Total RNA was purified by using RNeasy Miniprep kit (Qiagen, Chatsworth, CA), and 3 μg of RNA was run on a 1% agarose gel and transferred onto a positively charged nylon membrane (Roche Molecular Biochemicals). Hybridization probes for COX-2 and β-actin were synthesized by using their cDNA as templates for PCR and labeled with digoxigenin-11-dUTP (Roche Molecular Biochemicals). The mRNAs on the membrane were detected by chemiluminescent kit (Roche Molecular Biochemicals) and quantified by imagequant.
Statistical Analysis.
The unpaired Student's t test was used in the statistical analysis. All results are expressed as means ± SDs.
Results
Differential Effects of αT, γT, and γ-CEHC on PGE2 Generation in RAW264.7 Cells Stimulated with LPS and Epithelial Cells Treated with IL-1β.
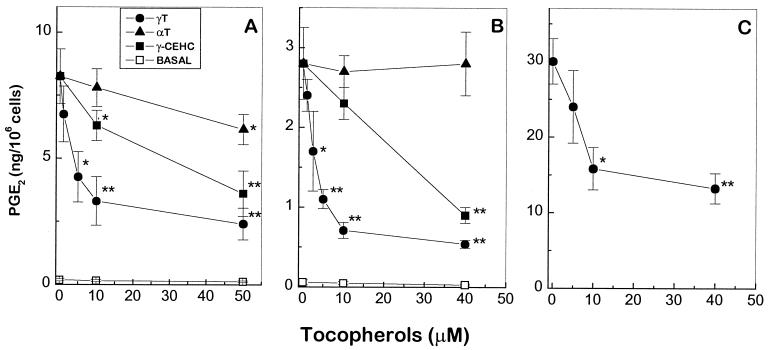
A marked increase of PGE2 generation was observed in RAW264.7 macrophage when cells were treated with 0.1 μg/ml LPS. Supplementation with γT led to a dose-dependent reduction of PGE2 synthesis (Fig. 1A), with an apparent IC50 of 7.5 ± 2 μM. In contrast to γT, αT with concentrations of less than 10 μM had little effect on PGE2 production, whereas 10 μM γ-CEHC showed ≈ 20% inhibition. At 50 μM, γT and γ-CEHC led to 70–80% and 60% reduction of PGE2 synthesis, respectively, whereas αT showed only ≈25% inhibition.
Figure 1.
Differential effects of αT, γT, and γ-CEHC on PGE2 synthesis in macrophage and epithelial cells. (A) RAW264.7 cells were preincubated with αT, γT, or vehicle, ethanol (0.1–0.2%) for 8–14 h in 0.5% FBS-DMEM and then treated with LPS (0.1 μg/ml) for 14 h. γ-CEHC was added 1 h before LPS treatment. (B) A549 epithelial cells were preincubated with αT, γT, or ethanol (0.1–0.2%) for 8–14 h and then treated with IL-1β (10 ng/ml) for 24 h. γ-CEHC was added 1 h before IL-1β treatment. (C) A549 cells were treated with γT and IL-1β as described in B. After IL-1β treatment, the supernatant was removed and cells were resuspended with fresh medium containing 10 μM AA for 10 min at 37°C. The supernatant was collected and PGE2 was measured as described in Materials and Methods. Basal was the amount of PGE2 produced from cells not treated with LPS or IL-1β. *, P < 0.05 and **, P < 0.01, control vs. tocopherol supplementation.
A549, a human lung epithelial cancer cell line, has been used to evaluate the anti-inflammatory effect of nonsteroidal anti-inflammatory drugs in intact cells (24). In IL-1β-treated A549 cells, both γT and γ-CEHC exhibited dose-dependent inhibition of PGE2 release, with IC50 of 4 ± 1 μM and 30 ± 3 μM, respectively (Fig. 1B). On the other hand, αT did not show any effect even at a concentration of 40 μM. The inhibitory effect of γT was also observed in the presence of exogenous AA (Fig. 1C).
Maximum inhibition of PGE2 was achieved when cells were preincubated with γT or αT for 8–14 h before LPS or IL-1β treatment, which is likely attributable to their slow cellular incorporation (25). After preincubation, washing the cells to remove free γT before the treatment only slightly diminished its inhibitory potency. In contrast, preincubation is not required for the optimal inhibition rendered by γ-CEHC and its effect was completely abolished by washing. This difference may be explained by distinct cellular retention that is associated with the hydrophobicity of the γT molecule in contrast to the more hydrophilic γ-CEHC. We also found that the inhibitory potency for γT was affected by the serum amount in the medium during LPS treatment. PGE2 formation was ≈ 10–20% more potently inhibited by γT when cells were treated with LPS in the medium containing 0.5% FBS compared with those in 10% FBS (data not shown).
The difference between γT and αT in inhibiting PGE2 synthesis is likely not caused by their differential cellular incorporation. Under our experimental conditions, both tocopherols were similarly absorbed by macrophages and the apparent γT incorporation was ≈30% higher than αT in human epithelial cells (see Materials and Methods).
Effect on Other Arachidonate Metabolites.
Consistent with a previous report (26), we found that besides PGE2, LPS-treated RAW264.7 macrophages generated substantial amounts of PGD2 (Table 1). In vitro supplementation of γT also led to reduction of PGD2 synthesis, although the inhibition of PGE2 was slightly but significantly more potent. This suggests that γT mainly affects the common step in the synthesis of different prostaglandins. Similar to the case with PGE2, αT had no effect on PGD2 release at 10 μM.
Table 1.
Effects of αT and γT on the generation of different AA metabolites in LPS-treated macrophages
| AA metabolite | Amount released, ng/106 cells
|
||
|---|---|---|---|
| Control | αT (10 μM) | γT (10 μM) | |
| PGE2 | 8.3 ± 1.1 | 7.8 ± 0.9 | 3.5 ± 1.0** |
| PGD2 | 264 ± 28 | 248 ± 15 | 129 ± 25** |
| 8-Isoprostane | 7.4 ± 0.7 | 5.6 ± 0.4* | 2.9 ± 0.6** |
RAW264.7 cells were preincubated with vehicle (control), αT, or γT for 14 h and treated with 0.1 μg/ml LPS for 14 h. AA metabolites released in the medium were measured. *, P < 0.05 and **, P < 0.01, controls vs. γT or αT supplementation.
Another AA metabolite, 8-isoprostane, has been recognized as a sensitive and specific marker of lipid peroxidation under oxidative stress (27). LPS stimulation of macrophage led to a marked increase in the release of 8-isoprostane. Supplementation with γT or αT caused significant reduction of isoprostane formation, and γT is significantly more potent than αT (Table 1), ≈60 and 25% reduction by 10 μM γT and αT, respectively.
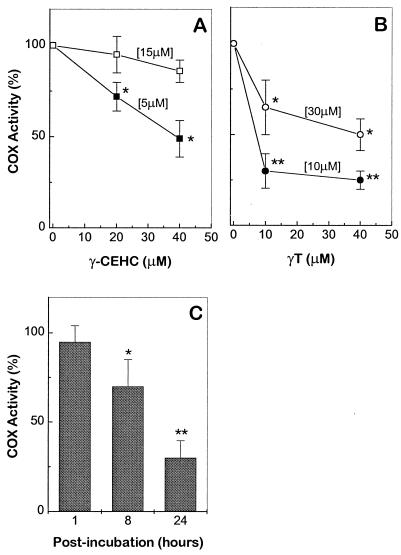
Inhibitory Effect of γT and γ-CEHC on COX-2 Activity in IL-1β-Pretreated Cells.
The ability of γT and γ-CEHC to directly inhibit COX-2 activity was tested after a 1-h exposure period in COX-2 preinduced epithelial cells, followed by the addition of exogenous AA. Under these conditions, γ-CEHC showed direct inhibition of PGE2 but no inhibitory effect for γT was observed (Fig. 2). However, if the exposure period for γT was extended to 8 or 24 h, the inhibition was evident (Fig. 2C). Their inhibitory potency declined as AA concentrations were increased (Fig. 2 A and B), suggesting that γT and γ-CEHC might compete with AA at the active site of COX-2. COX-2 protein levels were not affected by the postincubation with γT (data not shown).
Figure 2.
Inhibition of COX activity by postincubation with γT and γ-CEHC in COX-2-preinduced A549 cells. A549 cells were pretreated with IL-1β (10 ng/ml) for 24 h. (A) Cells were washed and incubated with fresh medium containing vehicle or γ-CEHC for 1 h. AA at final concentrations of 5 or 15 μM was added and incubated at 37°C for 10 min. (B) For γT, the postincubation period was extended to 24 h. Medium was then removed and replaced with a fresh one containing 10 or 30 μM AA at 37°C for 10 min. (C) Cells were postincubated with γT (10 μM) for 1, 8, or 24 h and COX-2 activity was measured as described in B. All reactions were stopped by the addition of 0.5 mM aspirin and PGE2 in the medium was measured. COX activity (%) is expressed as the ratio of PGE2 produced in the presence of γT or γ-CEHC to that with vehicle alone. Numbers within brackets indicate the final concentrations of AA. *, P < 0.05 and **, P < 0.01, control vs. tocopherol supplementation.
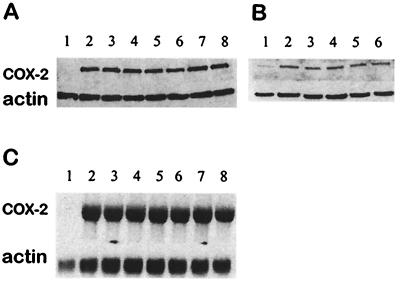
Effects on COX-2 Expression.
A significant increase in COX-2 expression was observed in LPS-treated macrophages or IL-1β-stimulated epithelial cells compared with untreated cells. Up to 50 μM, αT, γT, or γ-CEHC did not show significant inhibition of COX-2 protein expression (Fig. 3 A and B). Consistently, Northern blots showed that the induction of COX-2 mRNA was not affected by in vitro supplementation of tocopherols (Fig. 3C).
Figure 3.
Effects on COX-2 expression. (A) Western blot of RAW264.7 macrophages. Cells were treated with vehicle (lane 1); LPS (0.1 μg/ml, lane 2); or LPS (0.1 μg/ml) and γT (10 μM, lane3; and 40 μM, lane 4); αT (10 μM, lane 5; and 40 μM, lane 6) or γ-CEHC (10 μM, lane7; and 40 μM, lane 8) for 14 h. (B) Western blot of A549 cells; cells were treated with vehicle (lane 1); IL-1β (10 ng/ml, lane 2); or IL-1β and γT (10 μM, lane 3; and 40 μM, lane 4), and γ-CEHC (10 μM, lane 5; and 40 μM, lane 6) for 24 h. (C) Northern blot of RAW264.7 macrophages. The sample sequence was the same as specified in A.
Effects on Nitrite Release and iNOS Expression.
Nitrite accumulation was monitored to evaluate the potential effect of tocopherols on NOx formation in LPS-treated macrophages. At 10 μM, γT slightly inhibits (≈18%, P < 0.05) nitrite release (Table 2), whereas neither αT nor γ-CEHC significantly affect its accumulation. At 40–50 μM, the effect of αT, γT, and γ-CEHC was varied in four independent experiments (data not shown). Because nitric oxide has been shown to potentiate PGE2 synthesis in LPS-treated macrophages (28), we used NG-monomethyl-l-arginine (l-NMMA), a specific inhibitor of iNOS, to test whether NO has any effect on γT-exerted PGE2 inhibition. Under our experimental conditions, the blocking of NO formation by 1 mM l-NMMA did not affect PGE2 synthesis or the inhibitory potency of γT (Table 2), indicating that PGE2 formation is probably not mediated by NO.
Table 2.
Effect of tocopherols on nitrite accumulation and iNOS expression
| Treatment | Nitrite
release, μM
|
PGE2, ng/106
cells
|
iNOS expression,* % | ||
|---|---|---|---|---|---|
| Control | l-NMMA (1 mM) | Control | l-NMMA (1 mM) | ||
| LPS | 24.0 ± 3.1 | 2.1 ± 0.3† | 8.3 ± 1.1 | 8.4 ± 1.0 | 100 |
| γT (10 μM) | 19.4 ± 1.6* | 2.2 ± 0.2† | 3.5 ± 1.0** | 3.7 ± 0.8** | 69 ± 12** |
| αT (10 μM) | 22.1 ± 1.4 | ND | 7.8 ± 0.9 | ND | 76 ± 10* |
| γ-CEHC (10 μM) | 23.1 ± 2.6 | ND | 6.5 ± 0.6* | ND | 96 ± 8 |
RAW264.7 cells were preincubated with vehicle and various tocopherols for 14 h and then treated with 0.1 μg/ml LPS in the presence or absence (controls) of 1 mM NG-monomethyl-l-arginine (l-NMMA), for 14 h, at which time medium was collected and nitrite accumulation was measured. *, P < 0.05 and **, P < 0.01: vehicle vs. γT, αT, and γ-CEHC supplementation; †, P < 0.01: with vs. without 1 mM l-NMMA; ND, not determined.
iNOS (%) was obtained, using the Western blot, by the comparison of iNOS induced in the presence of various tocopherols with that expressed with ethanol alone.
LPS treatment led to a marked increase in iNOS expression in macrophages. Both αT and γT inhibited iNOS expression at 10 μM (Table 2), although αT at this concentration did not significantly suppress nitrite release.
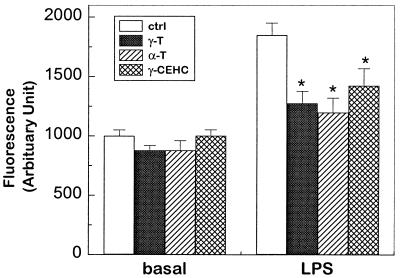
Inhibition of Reactive Oxygen Intermediates.
Oxidation of DCFH has been used to evaluate the total generation of reactive oxygen species including superoxide, hydrogen peroxide, and peroxynitrite (29). In RAW264.7 macrophages, LPS treatment caused a significant increase in fluorescent intensity compared with controls (P < 0.05, Fig. 4), indicating a marked generation of various oxidative species. Coincubation with αT, γT, and γ-CEHC led to the similar inhibition of DCFH oxidation, suggesting that they possess similar antioxidant activity.
Figure 4.
Total reactive oxygen species formation by DCFH assay. In 96-well plates, 6 × 104 cells per well were incubated for at least 4 h in the fresh medium containing 0.5% FBS supplemented with tocopherols or ethanol in controls. Cells were then treated with 0.1 μg/ml LPS for 3 h, washed twice with Hanks' balance saline solution, and finally incubated with 40 μM DCFH for 3 h or overnight. *, P < 0.05 control vs. tocopherol supplementation.
Discussion
Cyclooxygenase-catalyzed prostaglandin synthesis is one of the important early events during inflammation. As a key inflammatory mediator, PGE2 is known to stimulate cytokine generation (30), cause vasodilation, and mediate fever and pain (31). A major finding of our current study is that γT at physiologically relevant concentrations inhibits PGE2 generation in two independent cell lines. γT reduced PGE2 synthesis in a LPS-stimulated macrophage cell line, with an apparent IC50 of 7.5 μM, and in IL-1β-treated human epithelial cells, with an IC50 of 4 μM. The major metabolite of γT, γ-CEHC, exhibited a similar inhibitory effect with an IC50 of ≈30 μM in both cells. In contrast, αT at 50 μM only slightly reduced (25%) PGE2 formation in macrophages, but had no inhibitory effect in human epithelial cells. We also observed an inhibitory effect of γT and γ-CEHC on PGE2 generation in the primary human lung fibroblasts when treated with IL-1β (unpublished observation). These results demonstrate that γT and its major water-soluble metabolite possess anti-inflammatory properties.
The inhibitory effect of γT stems from its direct inhibition of cyclooxygenase activity in intact cells as shown by its inhibition (by 60–80%) of PGE2 synthesis and its lack of significant effect on COX-2 expression at concentrations of 10–50 μM. That the inhibition was observed in the presence of exogenous AA (Fig. 1C) demonstrates that the effect is not caused by a decrease in phospholipase A2 activity or substrate availability. γ-CEHC, but not γT, caused an immediate decline in PGE2 release after 1-h exposure to the IL-1β-pretreated A549 cells followed by addition of exogenous AA (Fig. 2). This is probably due to a more rapid cellular uptake of γ-CEHC, whereas the cellular incorporation of γT usually takes hours (25). Consistent with this hypothesis, when the incubation of γT with IL-1β-pretreated cells was extended to 8 or 24 h, which allowed substantial cellular incorporation, we observed an inhibitory effect (Fig. 2).
The effect of γT cannot be attributed to its antioxidant activity because both γT and αT exhibited similar ability to inhibit DCFH oxidation (Fig. 4), whereas αT showed a much less potent effect on PGE2 synthesis. When this paper was in preparation, Wu et al. (32) reported that in vitro supplementation of αT or γT in old mice cells caused similar inhibition of PGE2 synthesis. The discrepancy with the present study is likely due to the different systems used. It is known that compared with young cells, cells from old animals have much lower antioxidant capacity and significantly higher levels of lipid hydroperoxide (33, 34) that lead to an enhanced COX activity (35). Consequently, αT, a powerful antioxidant that decreases lipid hydroperoxide in old animals, reduced PGE2 release in old cells (35). Consistently, no inhibitory effect was observed when αT was supplemented in young rats (35, 36).
γT has a nonsubstituted 5-position, making it a better nucleophile for trapping electrophiles and a stronger inhibitor of peroxynitrite-induced lipid peroxidation (17) as compared with αT. Although peroxynitrite has been suggested as a peroxide source for COX-2 activity (26), in the current system, peroxynitrite is not likely to be a major mediator because PGE2 synthesis or inhibitory potency of γT was not affected by NO (Table 2). The physiological source of peroxide is thus unknown. A comprehensive understanding of the action of γT was also impeded by our unsuccessful attempt to test its inhibitory effect with the purified enzyme (data not shown). However, our results from intact cells suggest that γT and γ-CEHC may serve as weak competitive inhibitors of COX-2 because their inhibitory potency was diminished with an increase in AA concentration (Fig. 2). It remains to be determined whether the inhibitory activity of γT is caused by its competing with AA at the binding site of COX-2, or inhibition of lipid peroxidation as a nucleophile (17), or both.
Numerous studies demonstrate that nonsteroidal anti-inflammatory drugs exert their therapeutic effects by inhibiting COX activity (37). Drug potencies estimated in intact cells, compared with using purified enzyme, more accurately reflect their function as COX inhibitors in vivo (24, 38). In the present study, γT served as a COX inhibitor in intact cells but not with the purified enzyme, similarly to sodium salicylate, which inhibited cellular PGE2 synthesis with an IC50 of 25 μM but did not show inhibition with the purified COX-2 (24). Although further experiments are needed to evaluate γT therapeutic potency in vivo, the current finding is consistent with our recent observation that γT supplementation attenuated the inflammation-induced damage in rats (Q.J., J. Lykkesfeldt, E. T. Shigeno, B.N.A., M. K. Shigenaga, and S. Christen, unpublished data).
Although 8-isoprostane was originally proposed as a marker of lipid peroxidation generated independent of COX (39), several recent studies suggest it might be produced via a COX-catalyzed pathway under certain conditions, for instance, in LPS-treated human monocytes (40) or cytokine-activated smooth muscle cells (41). In the present study, γT, compared with αT, was more inhibitory to the isoprostane increase in LPS-treated macrophages (Table 1). This is in line with a COX-dependent mechanism, because γT is a COX inhibitor but αT is a better antioxidant. Consistent with this notion, we also observed a marked decline in isoprostane in the presence of indomethacin (data not shown). Although neither αT nor γT apparently affected COX-2 protein expression, they suppressed iNOS induction (Table 2), which, together with their similar inhibition of DCFH oxidation, confirms that both tocopherols were efficiently incorporated in the cells. Further experiments are needed to understand the mechanism of iNOS inhibition.
γT concentrations in human plasma vary from 1 to 6–7 μM (42), depending on the amount of dietary intake. These levels are comparable to the γT IC50 values (4–10 μM) estimated here in intact cells, and thus seem physiologically relevant. Although γT compared with αT is 4–5 times more abundant in diets (5), there have been few measurements of γT in human tissues and these have been mostly confined to adipose tissues (43, 44). Burton et al. (42) have recently reported that γT constitutes 30–50% of total vitamin E levels in human skin, muscle, and adipose tissues. The concentrations of γT in those tissues, e.g., 107 and 180 nmol/g (≈107 and 180 μM) of human muscle and skin, respectively (42), are remarkably higher than those measured in the corresponding rodent tissues, e.g., 3.5 nmol/g of rat muscle (6) and 3–4 nmol/g of mouse skin (45). This indicates that human retention of γT may be different from rodents. The potential role of γT in humans, therefore, may be unappreciated based on the experiments performed in rodents. More measurements on human tissue γT are required. Recent evidence indicates that as much as 50% of dietary γT may be catabolized to γ-CEHC (46), a hydrophilic product possessing the same chromanoxyl ring and a shorter phytyl chain with a carboxylate tail. γ-CEHC is recognized as a natriuretic factor in human urine (21) and its plasma concentration is ≈50–85 nM (47). Its level may be high in certain tissues such as kidney or increased with high dietary γT intake. We show that γ-CEHC is a COX-2 inhibitor with an apparent IC50 of 30 μM, which may be transiently achieved in tissues (such as the kidney), based on its urinary concentrations (46).
Our current findings that γT and its metabolite γ-CEHC possess anti-inflammatory properties may have important physiological implications. First, various animal and human tumor tissues, including human colon cancer, have been reported to contain enhanced COX-2 expression and PGE2 (48). PGE2 has been shown to promote proliferation in certain cancer cells and nonsteroidal anti-inflammatory drugs can inhibit the growth of carcinoma cells and suppress angiogenesis (48). Consequently, several population-based studies have detected a 40–50% decrease in relative risk for colorectal cancer in individuals who regularly use aspirin and other nonsteroidal anti-inflammatory drugs (49–51). Cooney et al. (16) report that γT is superior to αT in preventing neoplastic transformation in C3H 10T½ fibroblasts. Although the mechanism behind this effect is not fully understood, we propose that the ability of γT to inhibit PGE2 generation revealed in this study may be partially responsible for this effect.
In addition, inflammation plays a key role in the initiation and development of atherosclerosis and augmented expression of COX-2 has been found in human atherosclerotic lesions but not in normal arteries (52). The anti-inflammatory properties of γT may be important in preventing cardiovascular disease. Two clinical studies reported that serum levels of γT, but not αT, were reduced in coronary heart disease patients (11, 12). In one of the epidemiological studies, Kushi et al. (53) found that the intake of vitamin E from diets, which contain mostly γT, but not supplements, which consist predominantly of αT, was inversely correlated with death from coronary heart diseases. On the other hand, studies by Stampfer et al. (9), though not directly comparable, did not observe the same effect. The recent animal studies by Saldeen et al. (54) indicate that γT supplementation in Sprague–Dawley rats, compared with αT, showed more potent ex vivo inhibition of low density lipoprotein oxidation, platelet aggregation, and arterial thrombogenesis.
In summary, our current study demonstrates that γT and its major metabolite, but not αT, inhibit COX activity and thus possess anti-inflammatory activity. Our data combined with the cited human and animal studies suggest that γT may be important in public health. It may be that the inclusion of both αT and γT in vitamin E supplements is more effective in human disease prevention, especially considering that αT supplementation depresses γT in human plasma and adipose tissue (55).
Acknowledgments
We thank Drs. L. J. Marnett, S. Christen, M. K. Shigenaga, S. L. Hazen, and R. Stocker for critical reviews of this manuscript. This work was supported by a Postdoctoral Fellowship from the American Heart Association-Western Affiliates Grant 98–24 (Q.J.), the Wheeler Fund for the Biological Sciences at the University of California Berkeley, and the National Institute of Environmental Health Sciences Center Grant ES01896 (B.N.A.).
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenase
- DCFH
2′,7′-dichlorofluorescin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NOx
reactive nitrogen oxide species
- PGE2
prostaglandin E2
- PGD2
prostaglandin D2
- αT
α-tocopherol
- γT
γ-tocopherol
- γ-CEHC
2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200357097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200357097
References
- 1.Christen S, Hagen T M, Shigenaga M K, Ames B N. In: Microbes and Malignancy: Infection as a Cause of Human Cancers. Parsonnet J, editor. New York: Oxford Univ. Press; 1999. pp. 35–88. [Google Scholar]
- 2.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCall M R, Frei B. Free Radical Biol Med. 1999;26:1034–1053. doi: 10.1016/s0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- 4.Brigelius-Flohe R, Traber M G. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 5.Lehmann J, Martin H L, Lashley E L, Marshall M W, Judd J T. J Am Diet Assoc. 1986;86:1208–1216. [PubMed] [Google Scholar]
- 6.Bieri J G, Evarts R P. Am J Clin Nutr. 1974;27:980–986. doi: 10.1093/ajcn/27.8.980. [DOI] [PubMed] [Google Scholar]
- 7.Behrens W A, Madère R. J Am Coll Nutr. 1986;5:91–96. doi: 10.1080/07315724.1986.10720116. [DOI] [PubMed] [Google Scholar]
- 8.Bieri J G, Evarts R P. J Nutr. 1974;104:850–857. doi: 10.1093/jn/104.7.850. [DOI] [PubMed] [Google Scholar]
- 9.Gey K F, Puska P, Jordan P, Moser U K. Am J Clin Nutr. 1991;53:326S–334S. doi: 10.1093/ajcn/53.1.326S. [DOI] [PubMed] [Google Scholar]
- 10.Stampfer M J, Hennekens C H, Manson J E, Colditz G A, Rosner B, Willett W C. N Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 11.Ohrvall M, Sundlof G, Vessby B. J Intern Med. 1996;239:111–117. doi: 10.1046/j.1365-2796.1996.410753000.x. [DOI] [PubMed] [Google Scholar]
- 12.Kontush A, Spranger T, Reich A, Baum K, Beisiegel U. Atherosclerosis. 1999;144:117–122. doi: 10.1016/s0021-9150(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 13.GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 14.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. [Google Scholar]
- 15.Kristenson M, Zieden B, Kucinskiene Z, Elinder L S, Bergdahl B, Elwing B, Abaravicius A, Razinkoviene L, Calkauskas H, Olsson A G. Br Med J. 1997;314:629–633. doi: 10.1136/bmj.314.7081.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooney R V, Franke A A, Harwood P J, Hatch-Pigott V, Custer L J, Mordan L J. Proc Natl Acad Sci USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christen S, Woodall A A, Shigenaga M K, Southwell-Keely P T, Duncan M W, Ames B N. Proc Natl Acad Sci USA. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooney R V, Harwood P J, Franke A A, Narala K, Sundström A-K, Berggren P-O, Mordan L J. Free Radical Biol Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 19.Hoglen N C, Waller S C, Sipes I G, Liebler D C. Chem Res Toxicol. 1997;10:401–407. doi: 10.1021/tx960200h. [DOI] [PubMed] [Google Scholar]
- 20.Murray E D, Jr, Wechter W J, Kantoci D, Wang W H, Pham T, Quiggle D D, Gibson K M, Leipold D, Anner B M. J Pharmacol Exp Ther. 1997;282:657–662. [PubMed] [Google Scholar]
- 21.Wechter W J, Kantoci D, Murray E D, Jr, D'Amico D C, Jung M E, Wang W H. Proc Natl Acad Sci USA. 1996;93:6002–6007. doi: 10.1073/pnas.93.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdon C P, Burton B A, Prior R L. Anal Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- 23.LeBel C P, Ischiropoulos H, Bondy S C. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J A, Saunders M, Barnes P J, Newton R, Belvisi M G. Mol Pharmacol. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 25.Tran K, Chan A C. Lipids. 1992;27:38–41. doi: 10.1007/BF02537056. [DOI] [PubMed] [Google Scholar]
- 26.Landino L M, Crews B C, Timmons M D, Morrow J D, Marnett L J. Proc Natl Acad Sci USA. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts L J, II, Morrow J D. Biochim Biophys Acta. 1997;1345:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D, Misko T P, Masferrer J L, Seibert K, Currie M G, Needleman P. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imrich A, Ning Y Y, Kobzik L. J Leukocyte Biol. 1999;65:499–507. doi: 10.1002/jlb.65.4.499. [DOI] [PubMed] [Google Scholar]
- 30.Williams J A, Shacter E. J Biol Chem. 1997;272:25693–25699. doi: 10.1074/jbc.272.41.25693. [DOI] [PubMed] [Google Scholar]
- 31.Vane J R. Adv Prostaglandin Thromboxane Res. 1976;2:791–801. [PubMed] [Google Scholar]
- 32.Wu D, Meydani M, Beharka A A, Serafini M, Martin K R, Meydani S N. Free Radical Biol Med. 2000;28:643–651. doi: 10.1016/s0891-5849(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 33.Hagen T M, Ingersoll R T, Lykkesfeldt J, Liu J, Wehr C M, Vinarsky V, Bartholomew J C, Ames A B. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 34.Lykkesfeldt J, Hagen T M, Vinarsky V, Ames B N. FASEB J. 1998;12:1183–1189. doi: 10.1096/fasebj.12.12.1183. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Mura C, Beharka A A, Han S N, Paulson K E, Hwang D, Meydani S N. Am J Physiol. 1998;275:C661–C668. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto W, Fujie K, Nishihira J, Handa H, Ueda N, Yamamoto S. Biochim Biophys Acta. 1996;1304:139–144. doi: 10.1016/s0005-2760(96)00114-2. [DOI] [PubMed] [Google Scholar]
- 37.Vane J R, Botting R M. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 38.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow J D, Awad J A, Kato T, Takahashi K, Badr K F, Roberts L J D, Burk R F. J Clin Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratico D, FitzGerald G A. J Biol Chem. 1996;271:8919–8924. doi: 10.1074/jbc.271.15.8919. [DOI] [PubMed] [Google Scholar]
- 41.Jourdan K B, Evans T W, Goldstraw P, Mitchell J A. FASEB J. 1999;13:1025–1030. doi: 10.1096/fasebj.13.9.1025. [DOI] [PubMed] [Google Scholar]
- 42.Burton G W, Traber M G, Acuff R V, Walters D N, Kayden H, Hughes L, Ingold K U. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 43.Handelman G J, Epstein W L, Peerson J, Spiegelman D, Machlin L J, Dratz E A. Am J Clin Nutr. 1994;59:1025–1032. doi: 10.1093/ajcn/59.5.1025. [DOI] [PubMed] [Google Scholar]
- 44.Parker R S. Am J Clin Nutr. 1988;47:33–36. doi: 10.1093/ajcn/47.1.33. [DOI] [PubMed] [Google Scholar]
- 45.Weber C, Podda M, Rallis M, Thiele J J, Traber M G, Packer L. Free Radical Biol Med. 1997;22:761–769. doi: 10.1016/s0891-5849(96)00346-2. [DOI] [PubMed] [Google Scholar]
- 46.Swanson J E, Ben R N, Burton G W, Parker R S. J Lipid Res. 1999;40:665–671. [PubMed] [Google Scholar]
- 47.Stahl W, Graf P, Brigelius-Flohe R, Wechter W, Sies H. Anal Biochem. 1999;275:254–259. doi: 10.1006/abio.1999.4312. [DOI] [PubMed] [Google Scholar]
- 48.Levy G N. FASEB J. 1997;11:234–247. [PubMed] [Google Scholar]
- 49.Thun M J, Namboodiri M M, Calle E E, Flanders W D, Heath C W., Jr Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 50.Smalley W E, DuBois R N. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E, Egan K M, Hunter D J, Stampfer M J, Colditz G A, Willett W C, Speizer F E. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 52.Schonbeck U, Sukhova G K, Graber P, Coulter S, Libby P. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kushi L H, Folsom A R, Prineas R J, Mink P J, Wu Y, Bostick R M. N Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 54.Saldeen T, Li D, Mehta J L. J Am Coll Cardiol. 1999;34:1208–1215. doi: 10.1016/s0735-1097(99)00333-2. [DOI] [PubMed] [Google Scholar]
- 55.Handelman G J, Machlin L J, Fitch K, Weiter J J, Dratz E A. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]