Abstract
AIMS—Apolipoprotein J (apoJ) and apolipoprotein E (apoE) are thought to contribute to amyloid formation in patients with Alzheimer's disease. The aim of this investigation was to discover whether or not these apolipoproteins associate with corneal amyloid in gelatinous drop-like corneal dystrophy (GDCD) and lattice corneal dystrophy type I (LCD-I). METHODS—Corneas from three eyes of three patients with GDCD and one eye of one patient with LCD-I were examined immunohistochemically using antibodies against apoJ and apoE. Two normal corneas were similarly examined. Tissue sections of brain from a patient with Alzheimer's disease were used as positive controls for the antibodies. For all negative controls, mouse IgG was used instead of the primary antibody. RESULTS—Intense apoJ and apoE immunoreactivities were found in congophilic amyloid deposits in GDCD and LCD-I. These deposits were located subepithelially in GDCD, and subepithelially and intrastromally in LCD-I. In GDCD, immunostaining of subepithelial amyloid with anti-apoJ was noticeably stronger than with anti-apoE. CONCLUSIONS—As in senile plaques in brain from a patient with Alzheimer's disease, apoJ and apoE co-localise with amyloid in corneas with GDCD and LCD-I.
Full Text
The Full Text of this article is available as a PDF (147.8 KB).
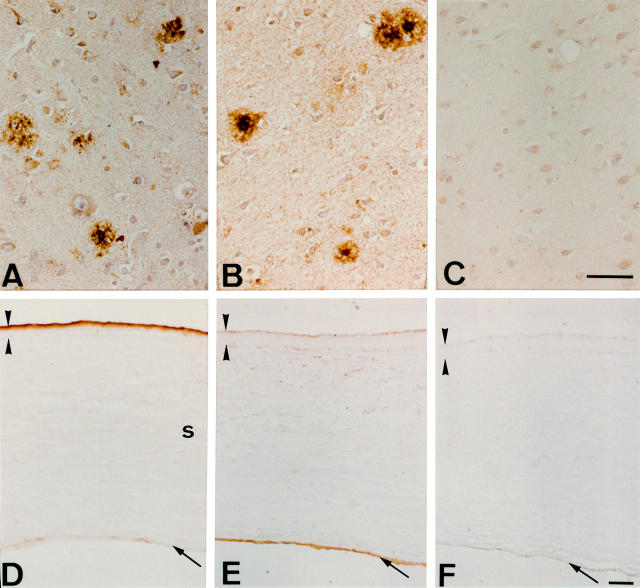
Figure 1 .
(A-C) Immunostaining of brain from a patient who had died of Alzheimer's disease with antibodies to (A) anti-apolipoprotein J and (B) anti-apolipoprotein E. Positive immunostaining is seen in senile plaques. (C) Control section incubated with normal mouse serum IgG shows no discernible immunolabelling. (D-F) Immunostaining of normal cornea with anti-apolipoprotein J and anti-apolipoprotein E antibodies. The epithelium is delineated by arrowheads, and the endothelium identified by an arrow; s = stroma. (D) The antibodies to apolipoprotein J stain the superficial epithelium (as was found previously21), whereas (E) the antibody to apolipoprotein E antibody does not stain epithelium. (E) Anti-apolipoprotein E appears to stain the endothelial/Descemet's membrane region, although at this time we cannot totally rule out the possibility that this might represent an edge artefact. (D, E) Diffuse staining for apolipoprotein J and apolipoprotein E was found in stroma. (F) Control section incubated with normal mouse serum IgG shows no discernible immunolabelling. Bar=50 µm (A-C) and 100 µm (D-F).
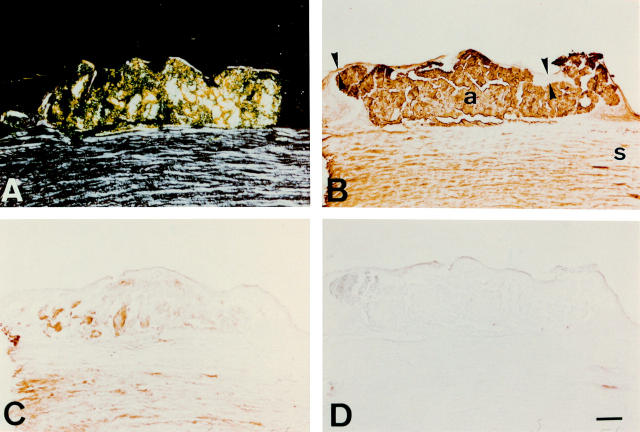
Figure 2 .
Immunostaining of gelatinous drop-like corneal dystrophy cornea with anti-apolipoprotein J and anti-apolipoprotein E antibodies. The epithelium (delineated by arrowheads in (B)) varies greatly in thickness. (A) When viewed under polarised light, congo red staining is seen in epithelial and subepithelial regions, showing a birefringent polarisation typical of amyloid. The posterior cornea is not visible here because tissue was obtained by a lamellar keratoplasty. (B) The antibodies to apolipoprotein J stain the subepithelial amyloid deposit (a) fairly intensely, and the stroma (s) more diffusely. (C) The anti-apolipoprotein E antibody also stains amyloid, but much less strongly than the anti-apolipoprotein J antibodies. (D) Control section incubated with normal mouse serum IgG shows no discernible immunolabelling. Bar=100 µm.
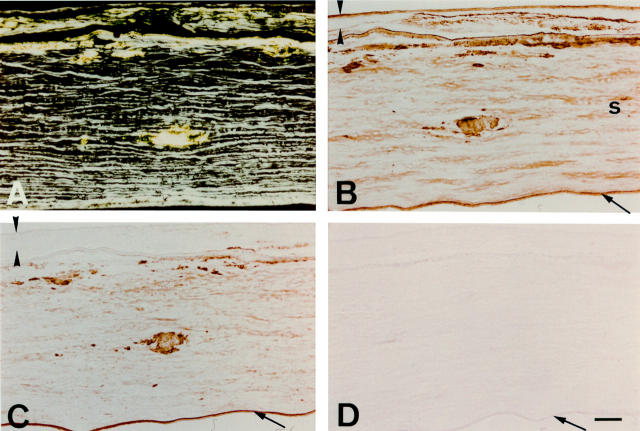
Figure 3 .
Immunostaining of a lattice corneal dystrophy type I cornea with anti-apolipoprotein J and anti-apolipoprotein E antibodies. The epithelium is delineated by arrowheads, and the endothelium identified by an arrow. (A) When viewed under polarised light, congo red staining is seen subepithelially and in corneal stroma, showing birefringent polarisation typical of amyloid. (B) Immunostaining for apolipoprotein J clearly mirrors the distribution of subepithelial and intrastromal amyloid deposits. (C) Apolipoprotein E immunoreactivity also co-exists with amyloid, but appears to stain the section less strongly than the apolipoprotein J antibodies. (D) Control section incubated with normal mouse serum IgG shows no discernible immunolabelling. Bar=100 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bales K. R., Verina T., Dodel R. C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E. M., Little S. P., Cummins D. J. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997 Nov;17(3):263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Choi-Miura N. H., Ihara Y., Fukuchi K., Takeda M., Nakano Y., Tobe T., Tomita M. SP-40,40 is a constituent of Alzheimer's amyloid. Acta Neuropathol. 1992;83(3):260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Tobe T., Hara K., Yoshida H., Tomita M. Sandwich ELISA assay for quantitative measurement of SP-40,40 in seminal plasma and serum. J Immunol Methods. 1990 Aug 7;131(2):159–163. doi: 10.1016/0022-1759(90)90186-y. [DOI] [PubMed] [Google Scholar]
- Dota A., Nishida K., Honma Y., Adachi W., Kawasaki S., Quantock A. J., Kinoshita S. Gelatinous drop-like corneal dystrophy is not one of the beta ig-h3-mutated corneal amyloidoses. Am J Ophthalmol. 1998 Dec;126(6):832–833. doi: 10.1016/s0002-9394(98)00186-x. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Matsubara E., Koudinov A., Choi-Miura N. H., Tomita M., Wisniewski T., Frangione B. The cerebrospinal-fluid soluble form of Alzheimer's amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993 Jul 1;293(Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gregory C. Y., Evans K., Bhattacharya S. S. Genetic refinement of the chromosome 5q lattice corneal dystrophy type I locus to within a 2 cM interval. J Med Genet. 1995 Mar;32(3):224–226. doi: 10.1136/jmg.32.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Hodge W. G., Damji K. F., Guernsey D. L., Neumann P. E. Lattice corneal dystrophy type 1 in a Canadian kindred is associated with the Arg124 --> Cys mutation in the kerato-epithelin gene. sgupta@ogh.on.ca. Am J Ophthalmol. 1998 Apr;125(4):547–549. doi: 10.1016/s0002-9394(99)80196-2. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Harr S. D., Uint L., Hollister R., Hyman B. T., Mendez A. J. Brain expression of apolipoproteins E, J, and A-I in Alzheimer's disease. J Neurochem. 1996 Jun;66(6):2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki S., Nishida K., Quantock A. J., Dota A., Bennett K., Kinoshita S. Amyloid and Pro501 Thr-mutated (beta)ig-h3 gene product colocalize in lattice corneal dystrophy type IIIA. Am J Ophthalmol. 1999 Apr;127(4):456–458. doi: 10.1016/s0002-9394(98)00360-2. [DOI] [PubMed] [Google Scholar]
- Kivelä T., Tarkkanen A., McLean I., Ghiso J., Frangione B., Haltia M. Immunohistochemical analysis of lattice corneal dystrophies types I and II. Br J Ophthalmol. 1993 Dec;77(12):799–804. doi: 10.1136/bjo.77.12.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth G. K., Sommer J. R., Obrian G., Han L., Ahmed M. N., Qumsiyeh M. B., Lin P. Y., Basti S., Reddy M. K., Kanai A. Familial subepithelial corneal amyloidosis (gelatinous drop-like corneal dystrophy): exclusion of linkage to lactoferrin gene. Mol Vis. 1998 Dec 31;4:31–31. [PubMed] [Google Scholar]
- Klintworth G. K., Valnickova Z., Kielar R. A., Baratz K. H., Campbell R. J., Enghild J. J. Familial subepithelial corneal amyloidosis--a lactoferrin-related amyloidosis. Invest Ophthalmol Vis Sci. 1997 Dec;38(13):2756–2763. [PubMed] [Google Scholar]
- Korvatska E., Munier F. L., Djemaï A., Wang M. X., Frueh B., Chiou A. G., Uffer S., Ballestrazzi E., Braunstein R. E., Forster R. K. Mutation hot spots in 5q31-linked corneal dystrophies. Am J Hum Genet. 1998 Feb;62(2):320–324. doi: 10.1086/301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Edward D. P., Ratnakar K. S., Reddy M., Tso M. O. Clinicohistopathological findings of gelatinous droplike corneal dystrophy among Asians. Cornea. 1996 Jul;15(4):355–362. doi: 10.1097/00003226-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara E., Frangione B., Ghiso J. Characterization of apolipoprotein J-Alzheimer's A beta interaction. J Biol Chem. 1995 Mar 31;270(13):7563–7567. doi: 10.1074/jbc.270.13.7563. [DOI] [PubMed] [Google Scholar]
- Munier F. L., Korvatska E., Djemaï A., Le Paslier D., Zografos L., Pescia G., Schorderet D. F. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997 Mar;15(3):247–251. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Adachi W., Shimizu-Matsumoto A., Kinoshita S., Mizuno K., Matsubara K., Okubo K. A gene expression profile of human corneal epithelium and the isolation of human keratin 12 cDNA. Invest Ophthalmol Vis Sci. 1996 Aug;37(9):1800–1809. [PubMed] [Google Scholar]
- Nishida K., Kawasaki S., Adachi W., Kinoshita S. Apolipoprotein J expression in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 1996 Oct;37(11):2285–2292. [PubMed] [Google Scholar]
- Oda T., Wals P., Osterburg H. H., Johnson S. A., Pasinetti G. M., Morgan T. E., Rozovsky I., Stine W. B., Snyder S. W., Holzman T. F. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neurol. 1995 Nov;136(1):22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- Quantock A. J., Nishida K., Kinoshita S. Histopathology of recurrent gelatinous drop-like corneal dystrophy. Cornea. 1998 Mar;17(2):215–221. doi: 10.1097/00003226-199803000-00018. [DOI] [PubMed] [Google Scholar]
- Reeder D. J., Stuart W. D., Witte D. P., Brown T. L., Harmony J. A. Local synthesis of apolipoprotein J in the eye. Exp Eye Res. 1995 May;60(5):495–504. doi: 10.1016/s0014-4835(05)80064-8. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Mathers W. D., Rosenwasser G. O., Holland E. J., Folberg R., Krachmer J. H., Nichols B. E., Gorevic P. D., Taylor C. M., Streb L. M. Three autosomal dominant corneal dystrophies map to chromosome 5q. Nat Genet. 1994 Jan;6(1):47–51. doi: 10.1038/ng0194-47. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács L., Boross P., Tözser J., Módis L., Jr, Tóth G., Berta A. Transforming growth factor-beta induced protein, betaIG-H3, is present in degraded form and altered localization in lattice corneal dystrophy type I. Exp Eye Res. 1998 Jun;66(6):739–745. doi: 10.1006/exer.1998.0471. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M., Kurahashi H., Tanaka T., Nishida K., Shimomura Y., Tano Y., Nakamura Y. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat Genet. 1999 Apr;21(4):420–423. doi: 10.1038/7759. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M., Kurahashi H., Tanaka T., Okada M., Yamamoto S., Maeda N., Watanabe H., Inoue Y., Kiridoshi A., Matsumoto K. Homozygosity mapping of a gene responsible for gelatinous drop-like corneal dystrophy to chromosome 1p. Am J Hum Genet. 1998 Oct;63(4):1073–1077. doi: 10.1086/302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Castaño E. M., Golabek A., Vogel T., Frangione B. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994 Nov;145(5):1030–1035. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A. A., Kida E., Wisniewski K. E., Frangione B. Conformational mimicry in Alzheimer's disease. Role of apolipoproteins in amyloidogenesis. Am J Pathol. 1995 Aug;147(2):238–244. [PMC free article] [PubMed] [Google Scholar]