Abstract
AIM—To examine in vitro whether phosphorylcholine coating of poly(methylmethacrylate) can reduce the adhesion of fibrinogen, fibrin, human scleral fibroblast and macrophage compared with current biomaterials used in the construction of glaucoma drainage devices. METHODS—Sample discs (n=6) of poly(methylmethacrylate), silicone, polypropylene, PTFE, and phosphorylcholine coated poly(methylmethacrylate) were seeded with fibrinogen, fibrin, fibroblast, and macrophages and incubated for variable lengths of time. The quantification was performed using radioactivity, spectrophotometry, ATP dependent luminometry, and immunohistochemistry respectively. RESULTS—Fibrinogen and fibrin adhesion to phosphorylcholine coated poly(methylmethacrylate) were significantly lower than PMMA (p=0.004). Phosphorylcholine coating of poly(methylmethacrylate) also significantly reduced the adhesion of human scleral fibroblast (p=0.002) and macrophage (p=0.01) compared with PMMA. All the other biomaterials showed either similar or insignificantly different levels of adhesion to all the proteins and cells tested compared with PMMA. CONCLUSION—Phosphorylcholine coating is a new material technology that offers considerable promise in the field of glaucoma drainage device development.
Full Text
The Full Text of this article is available as a PDF (82.8 KB).
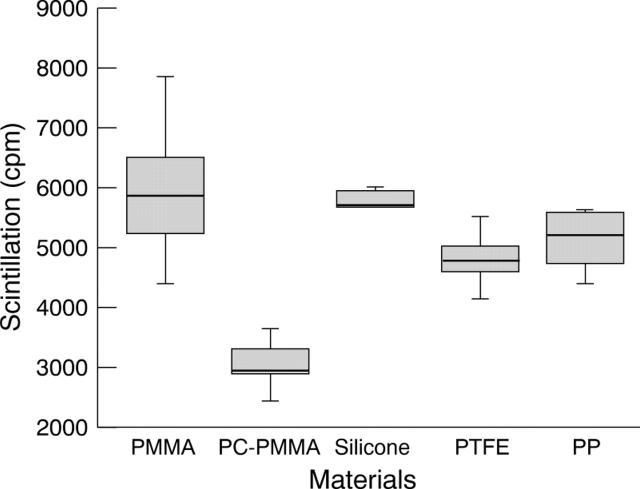
Figure 1 .
Adsorption of iodine-125 labelled fibrinogen to biomaterials after 2 hours' incubation in PBS.
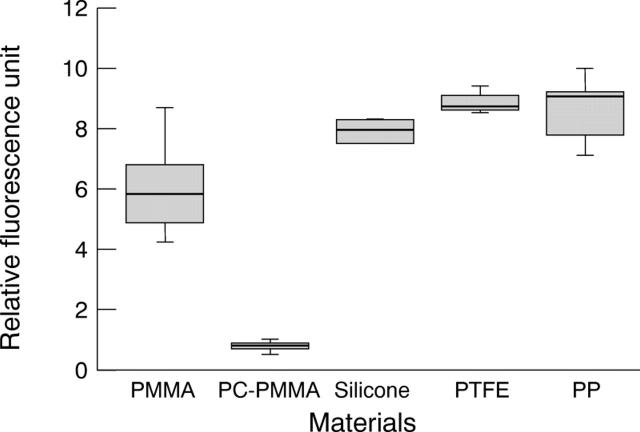
Figure 2 .
Adhesion of fibrin to biomaterials from fresh plasma after 2 hours' incubation.
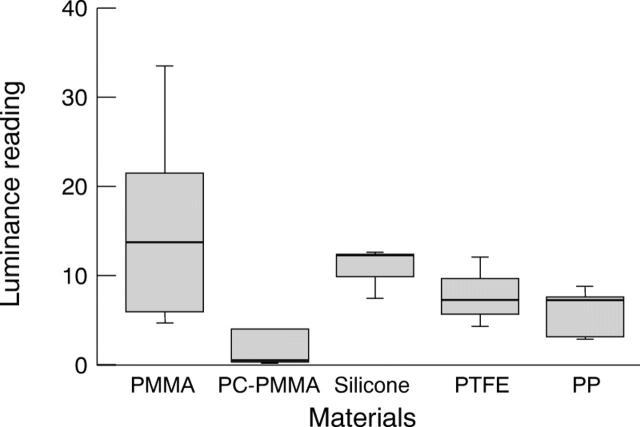
Figure 3 .
In vitro adhesion of human scleral fibroblast to biomaterials.
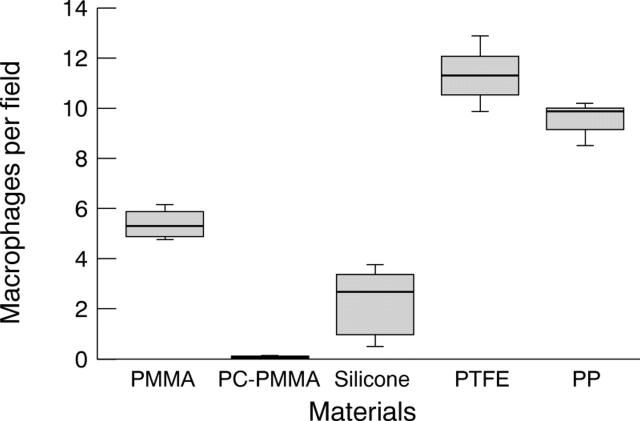
Figure 4 .
In vitro adhesion of macrophage to biomaterials.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins W. E., Mosher D. F., Tomasini B. R., Cooper S. L. A preliminary comparison of the thrombogenic activity of vitronectin and other RGD-containing proteins when bound to surfaces. Ann N Y Acad Sci. 1987;516:291–299. doi: 10.1111/j.1749-6632.1987.tb33049.x. [DOI] [PubMed] [Google Scholar]
- Durrani A. A., Hayward J. A., Chapman D. Biomembranes as models for polymer surfaces. II. The syntheses of reactive species for covalent coupling of phosphorylcholine to polymer surfaces. Biomaterials. 1986 Mar;7(2):121–125. doi: 10.1016/0142-9612(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Kojima M., Ishihara K., Watanabe A., Nakabayashi N. Interaction between phospholipids and biocompatible polymers containing a phosphorylcholine moiety. Biomaterials. 1991 Mar;12(2):121–124. doi: 10.1016/0142-9612(91)90189-h. [DOI] [PubMed] [Google Scholar]
- Kottke-Marchant K., Anderson J. M., Umemura Y., Marchant R. E. Effect of albumin coating on the in vitro blood compatibility of Dacron arterial prostheses. Biomaterials. 1989 Apr;10(3):147–155. doi: 10.1016/0142-9612(89)90017-3. [DOI] [PubMed] [Google Scholar]
- Larsson R., Selén G., Björdklund H., Fagerholm P. Intraocular PMMA lenses modified with surface-immobilized heparin: evaluation of biocompatibility in vitro and in vivo. Biomaterials. 1989 Oct;10(8):511–516. doi: 10.1016/0142-9612(89)90055-0. [DOI] [PubMed] [Google Scholar]
- Lee V. W. Glaucoma "valves"--truth versus myth. Ophthalmology. 1998 Apr;105(4):567–568. doi: 10.1016/S0161-6420(98)94000-3. [DOI] [PubMed] [Google Scholar]
- Lim K. S., Allan B. D., Lloyd A. W., Muir A., Khaw P. T. Glaucoma drainage devices; past, present, and future. Br J Ophthalmol. 1998 Sep;82(9):1083–1089. doi: 10.1136/bjo.82.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEWEN W. K. Application of Poiseuille's law to aqueous outflow. AMA Arch Ophthalmol. 1958 Aug;60(2):290–294. doi: 10.1001/archopht.1958.00940080306017. [DOI] [PubMed] [Google Scholar]
- Porter J. M., Krawczyk C. H., Carey R. F. In vitro flow testing of glaucoma drainage devices. Ophthalmology. 1997 Oct;104(10):1701–1707. doi: 10.1016/s0161-6420(97)30077-3. [DOI] [PubMed] [Google Scholar]
- Prata J. A., Jr, Mérmoud A., LaBree L., Minckler D. S. In vitro and in vivo flow characteristics of glaucoma drainage implants. Ophthalmology. 1995 Jun;102(6):894–904. doi: 10.1016/s0161-6420(95)30937-2. [DOI] [PubMed] [Google Scholar]
- Shahinian L., Jr, Egbert P. R., Williams A. S. Histologic study of healing after ab interno laser sclerostomy. Am J Ophthalmol. 1992 Aug 15;114(2):216–219. doi: 10.1016/s0002-9394(14)73988-1. [DOI] [PubMed] [Google Scholar]
- Smetana K., Jr, Lukás J., Palecková V., Bartůnkovä J., Liu F. T., Vacík J., Gabius H. J. Effect of chemical structure of hydrogels on the adhesion and phenotypic characteristics of human monocytes such as expression of galectins and other carbohydrate-binding sites. Biomaterials. 1997 Jul;18(14):1009–1014. doi: 10.1016/s0142-9612(97)00037-9. [DOI] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993 Dec 1;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Inflammatory responses to biomaterials. Am J Clin Pathol. 1995 Apr;103(4):466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- Tang L., Lucas A. H., Eaton J. W. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. J Lab Clin Med. 1993 Sep;122(3):292–300. [PubMed] [Google Scholar]