Full Text
The Full Text of this article is available as a PDF (155.9 KB).
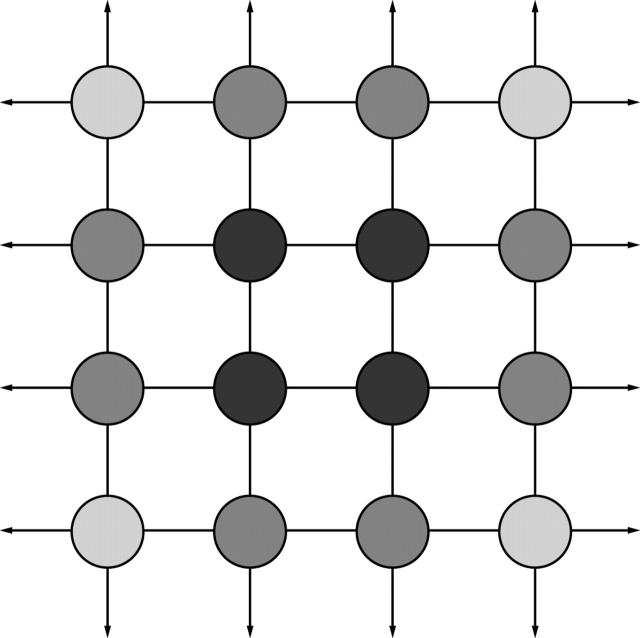
Figure 1 .
A simple two dimensional lattice illustrating surface reactivity. Molecules within the lattice are at a lower energy state (darker shade) than molecules at the surface which have more unoccupied bonding sites (arrows). Interfacial free energy is a measure of the number and reactivity of unoccupied bonding sites at the interface between a material surface and its surroundings. Polymers such as poly(tetrafluoroethylene) (PTFE, Teflon) have a have a relatively unreactive surface and are less prone to biological spoilation in aqueous systems than hydrophobic materials (for example, silicone, PMMA) with a higher interfacial free energy.
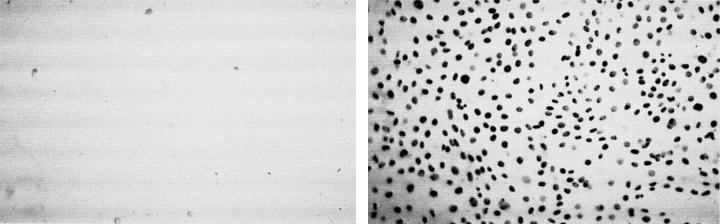
Figure 2 .
3T3 fibroblasts cultured for 24 hours with bromodeoxyuridine (BRDU) to label new DNA shows cell adhesion and division is greatly reduced for phosphoryl choline copolymer coated (left) versus uncoated (right) poly(methylmethacrylate) (PMMA). (Courtesy of Dr Andrew Lloyd, University of Brighton.)
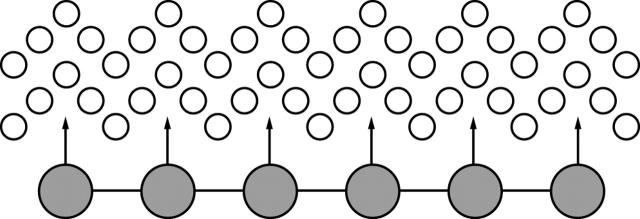
Figure 3 .
Hydrogels reduce their interfacial free energy in aqueous systems by trapping a shell of water molecules (open circles) which tend to shield their reactive domains (arrows). Bioinert polymers and natural cell surfaces may resist non-specific adsorption through this micromolecular exclusion zone.
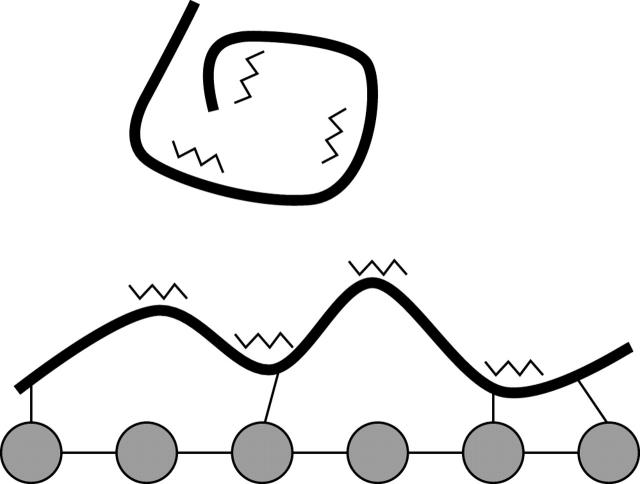
Figure 4 .
Adsorption of soluble adhesion molecules (thick lines) to a hydroxyapatite surface may induce a conformational change resulting in the exposure of previously sequestered integrin binding domains (jagged lines), promoting cell adhesion. The effect of incorporating integrin-ligand-peptide sequences on cell behaviour in synthetic matrices and artificial surfaces is currently being explored.
Figure 5 .
A scanning electron micrograph showing a human osteoblast reaching pseudopodia out to hydroxyapatite particles (lighter areas) dispersed within a bioactive ceramic-polymer composite (HAPEX) (scale bar = 10 µm). (Courtesy of Dr Lucy Di Silvio, The IRC in Biomedical Materials, University of London.)
Figure 6 .
A tissue engineered artificial cornea.55 Sheets of keratocytes cultured for 35 days produce a thick collagenous matrix. Two of these matrix/cell sheets are then peeled away from the culture dish, and superimposed to form a corneal stromal equivalent. In appropriate conditions, a multilayered corneal epithelium can be cultured on this stromal equivalent. These epithelial cells lay down a basement membrane containing type IV collagen, lamenin, and fibronectin. (Courtesy of Dr Patrick Carrier, LOEX Laboratory, Laval University, Quebec, Canada.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amon M., Menapace R. Cellular invasion on hydrogel and poly(methyl methacrylate) implants. An in vivo study. J Cataract Refract Surg. 1991 Nov;17(6):774–779. doi: 10.1016/s0886-3350(13)80410-5. [DOI] [PubMed] [Google Scholar]
- Ashworth J. L., Rhatigan M., Sampath R., Brammar R., Sunderland S., Leatherbarrow B. The hydroxyapatite orbital implant: a prospective study. Eye (Lond) 1996;10(Pt 1):29–37. doi: 10.1038/eye.1996.4. [DOI] [PubMed] [Google Scholar]
- Bell E., Ehrlich H. P., Buttle D. J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981 Mar 6;211(4486):1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Bjornson C. R., Rietze R. L., Reynolds B. A., Magli M. C., Vescovi A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999 Jan 22;283(5401):534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Cao Y., Vacanti J. P., Paige K. T., Upton J., Vacanti C. A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997 Aug;100(2):297–304. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Davis M. W., Vacanti J. P. Toward development of an implantable tissue engineered liver. Biomaterials. 1996 Feb;17(3):365–372. doi: 10.1016/0142-9612(96)85575-x. [DOI] [PubMed] [Google Scholar]
- Dee K. C., Andersen T. T., Bizios R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J Biomed Mater Res. 1998 Jun 5;40(3):371–377. doi: 10.1002/(sici)1097-4636(19980605)40:3<371::aid-jbm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dornhoffer J. L. Hearing results with the Dornhoffer ossicular replacement prostheses. Laryngoscope. 1998 Apr;108(4 Pt 1):531–536. doi: 10.1097/00005537-199804000-00013. [DOI] [PubMed] [Google Scholar]
- Durrani A. A., Hayward J. A., Chapman D. Biomembranes as models for polymer surfaces. II. The syntheses of reactive species for covalent coupling of phosphorylcholine to polymer surfaces. Biomaterials. 1986 Mar;7(2):121–125. doi: 10.1016/0142-9612(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M. The integrin superfamily and the eye. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):696–701. [PubMed] [Google Scholar]
- García A. J., Ducheyne P., Boettiger D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J Biomed Mater Res. 1998 Apr;40(1):48–56. doi: 10.1002/(sici)1097-4636(199804)40:1<48::aid-jbm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Grimson W. E., Kikinis R., Jolesz F. A., Black P. M. Image-guided surgery. Sci Am. 1999 Jun;280(6):62–69. doi: 10.1038/scientificamerican0699-62. [DOI] [PubMed] [Google Scholar]
- Gristina A. G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Harrison D., Johnson R., Tucci M., Puckett A., Tsao A., Hughes J., Benghuzzi H. Interaction of cells with UHMWPE impregnated with the bioactive peptides RGD, RGE or Poly-L-lysine. Biomed Sci Instrum. 1997;34:41–46. [PubMed] [Google Scholar]
- Hayward J. A., Chapman D. Biomembrane surfaces as models for polymer design: the potential for haemocompatibility. Biomaterials. 1984 May;5(3):135–142. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- Humes H. D. Tissue engineering of a bioartificial kidney: a universal donor organ. Transplant Proc. 1996 Aug;28(4):2032–2035. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Oshida H., Endo Y., Ueda T., Watanabe A., Nakabayashi N. Hemocompatibility of human whole blood on polymers with a phospholipid polar group and its mechanism. J Biomed Mater Res. 1992 Dec;26(12):1543–1552. doi: 10.1002/jbm.820261202. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Ziats N. P., Tierney B. P., Nakabayashi N., Anderson J. M. Protein adsorption from human plasma is reduced on phospholipid polymers. J Biomed Mater Res. 1991 Nov;25(11):1397–1407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- Keller G., Snodgrass H. R. Human embryonic stem cells: the future is now. Nat Med. 1999 Feb;5(2):151–152. doi: 10.1038/5512. [DOI] [PubMed] [Google Scholar]
- L'Heureux N., Pâquet S., Labbé R., Germain L., Auger F. A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998 Jan;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- Langer R., Vacanti J. P. Tissue engineering. Science. 1993 May 14;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996 Feb 9;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- León C. R., Barraquer J. I., Jr, Barraquer J. I., Sr Coralline hydroxyapatite keratoprosthesis in rabbits. J Refract Surg. 1997 Jan-Feb;13(1):74–78. doi: 10.3928/1081-597X-19970101-16. [DOI] [PubMed] [Google Scholar]
- Lobel K. D., Hench L. L. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J Biomed Mater Res. 1998 Mar 15;39(4):575–579. doi: 10.1002/(sici)1097-4636(19980315)39:4<575::aid-jbm11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Naughton G., Mansbridge J., Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs. 1997 Nov;21(11):1203–1210. doi: 10.1111/j.1525-1594.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Matsuura T., Hosokawa R., Akagawa Y. RGD peptides regulate the specific adhesion scheme of osteoblasts to hydroxyapatite but not to titanium. J Dent Res. 1998 Mar;77(3):481–487. doi: 10.1177/00220345980770030701. [DOI] [PubMed] [Google Scholar]
- Okano T., Matsuda T. Tissue engineered skeletal muscle: preparation of highly dense, highly oriented hybrid muscular tissues. Cell Transplant. 1998 Jan-Feb;7(1):71–82. doi: 10.1177/096368979800700110. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Schaller M. D., Ginsberg M. H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sittinger M., Bujia J., Rotter N., Reitzel D., Minuth W. W., Burmester G. R. Tissue engineering and autologous transplant formation: practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials. 1996 Feb;17(3):237–242. doi: 10.1016/0142-9612(96)85561-x. [DOI] [PubMed] [Google Scholar]
- Strong A. B., Stubley G. D., Chang G., Absolom D. R. Theoretical and experimental analysis of cellular adhesion to polymer surfaces. J Biomed Mater Res. 1987 Aug;21(8):1039–1055. doi: 10.1002/jbm.820210810. [DOI] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993 Dec 1;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Inflammatory responses to biomaterials. Am J Clin Pathol. 1995 Apr;103(4):466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- Tang L., Lucas A. H., Eaton J. W. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. J Lab Clin Med. 1993 Sep;122(3):292–300. [PubMed] [Google Scholar]
- Yannas I. V., Burke J. F., Orgill D. P., Skrabut E. M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982 Jan 8;215(4529):174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- Young G., Bowers R., Hall B., Port M. Clinical comparison of Omafilcon A with four control materials. CLAO J. 1997 Oct;23(4):249–258. [PubMed] [Google Scholar]
- Young G., Bowers R., Hall B., Port M. Six month clinical evaluation of a biomimetic hydrogel contact lens. CLAO J. 1997 Oct;23(4):226–236. [PubMed] [Google Scholar]
- Zamet J. S., Darbar U. R., Griffiths G. S., Bulman J. S., Brägger U., Bürgin W., Newman H. N. Particulate bioglass as a grafting material in the treatment of periodontal intrabony defects. J Clin Periodontol. 1997 Jun;24(6):410–418. doi: 10.1111/j.1600-051x.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]