Abstract
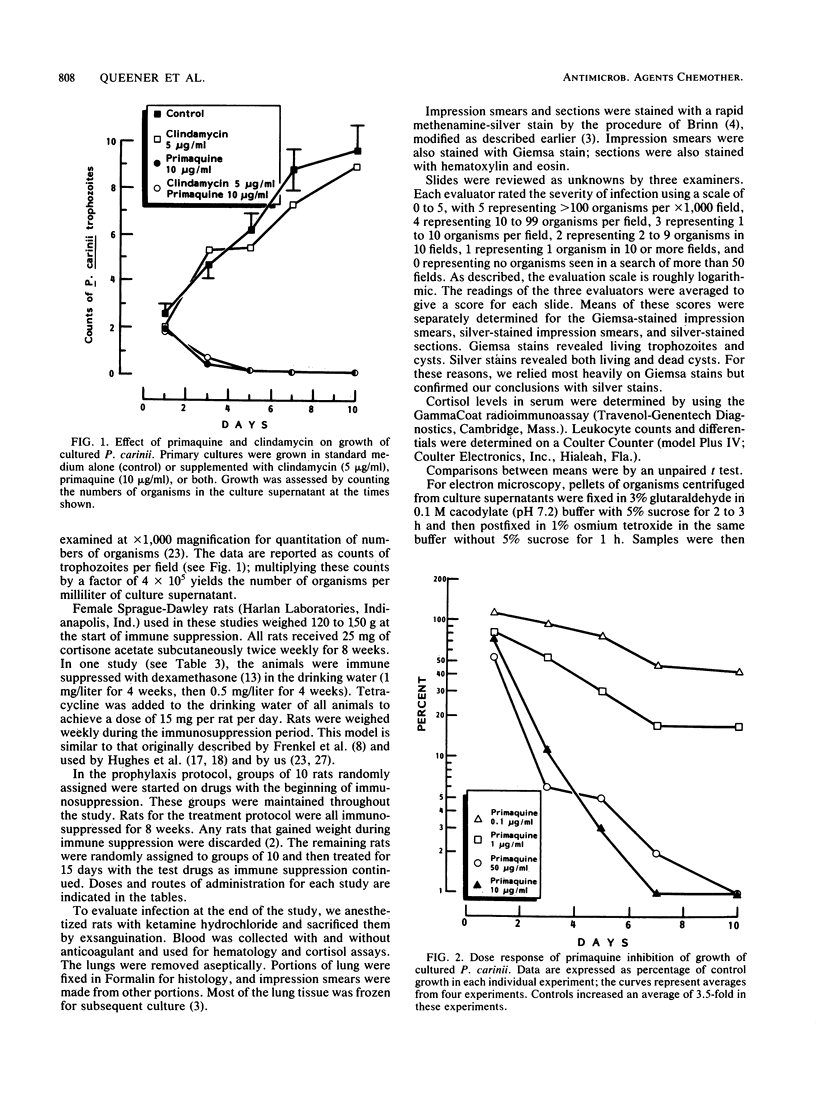
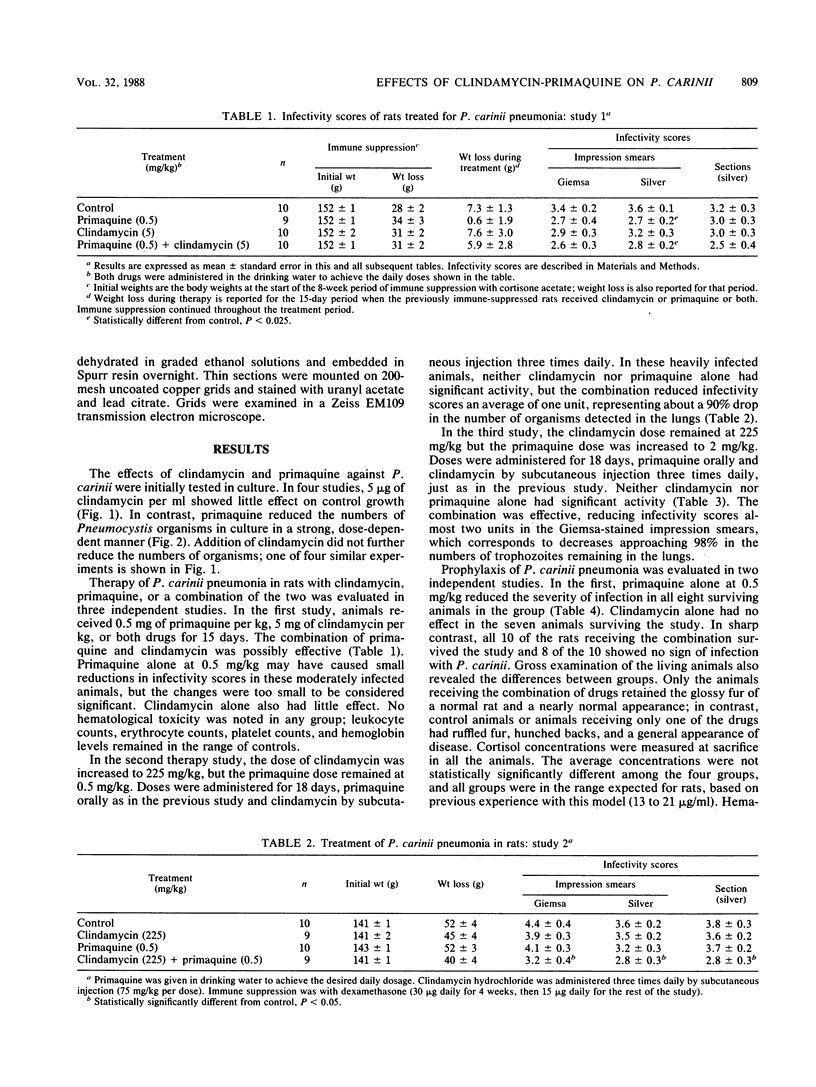
The combination of primaquine with clindamycin is effective in both in vitro and in vivo models of Pneumocystis infection. Primaquine alone at concentrations from 10 to 300 micrograms/ml reduced the numbers of organisms in cultures to less than 7% of control. Significant inhibition was observed down to 0.1 microgram/ml. Clindamycin at 5 micrograms/ml was ineffective alone. Combinations of clindamycin and primaquine in culture at various concentrations were effective, but there was no evidence of true synergy. In rats with established Pneumocystis pneumonia, clindamycin alone at 5 or 225 mg/kg was ineffective. Primaquine alone at 0.5 or 2 mg/kg did not significantly affect the numbers of organisms remaining. The combination of 0.5 mg of primaquine per kg and 225 mg of clindamycin per kg was effective for therapy, lowering the numbers of organisms in the lungs by about 90%. The combination of 2 mg of primaquine per kg and 225 mg of clindamycin per kg was more effective, lowering the numbers of organisms by almost 98%. In the in vivo prophylaxis model, primaquine at 0.1 or 0.2 mg/kg did not prevent the development of Pneumocystis pneumonia in immune-suppressed rats. Clindamycin at 50 mg/kg had a modest effect alone, but at 5 mg/kg all animals became heavily infected. At 0.5 mg/kg, primaquine alone reduced the severity of infection, but seven of eight rats were still infected. In contrast, the combination of 5 mg of clindamycin per kg and 0.5 mg of primaquine per kg prevented infection in 8 of 10 rats; 2 rats had minimal infection. These studies suggest that the combination of clindamycin and primaquine should be tested in therapy or prophylaxis of Pneumocystis infections in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Chabner B. A., Tuazon C. U., Ogata-Arakaki D., Baird B., Drake J. C., Simmons J. T., Lack E. E., Shelhamer J. H., Balis F. Trimetrexate for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1987 Oct 15;317(16):978–985. doi: 10.1056/NEJM198710153171602. [DOI] [PubMed] [Google Scholar]
- Bartlett M. S., Queener S. F., Jay M. A., Durkin M. M., Smith J. W. Improved rat model for studying Pneumocystis carinii pneumonia. J Clin Microbiol. 1987 Mar;25(3):480–484. doi: 10.1128/jcm.25.3.480-484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo A. A., Geary T. G., Jensen J. B. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1985 Jan;27(1):21–27. doi: 10.1128/aac.27.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H., Hong J. J., Ogra P. L. In-vivo effects of clindamycin on neutrophil function. J Antimicrob Chemother. 1985 Nov;16(5):649–657. doi: 10.1093/jac/16.5.649. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Golden J. A., Sjoerdsma A., Santi D. V. Pneumocystis carinii pneumonia treated with alpha-difluoromethylornithine. A prospective study among patients with the acquired immunodeficiency syndrome. West J Med. 1984 Nov;141(5):613–623. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M. S., Knight S., Mitsuyasu R., Weisman J., Roth M., Young L. S. Prophylaxis of Pneumocystis carinii infection in AIDS with pyrimethamine-sulfadoxine. Lancet. 1984 Aug 18;2(8399):398–399. doi: 10.1016/s0140-6736(84)90560-9. [DOI] [PubMed] [Google Scholar]
- Grewal R. S. Pharmacology of 8-aminoquinolines. Bull World Health Organ. 1981;59(3):397–406. [PMC free article] [PubMed] [Google Scholar]
- Hendley J. O., Weller T. H. Activation and transmission in rats of infection with Pneumocystis. Proc Soc Exp Biol Med. 1971 Sep;137(4):1401–1404. doi: 10.3181/00379727-137-35798. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Remington J. S. Clindamycin in a murine model of toxoplasmic encephalitis. Antimicrob Agents Chemother. 1987 Apr;31(4):492–496. doi: 10.1128/aac.31.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook D. J., Jr, Griffin J. B., Fowler L., Gibson B. R. Tissue distribution of primaquine in the rat. Pharmacology. 1981;22(5):330–336. doi: 10.1159/000137508. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., McNabb P. C., Makres T. D., Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974 Mar;5(3):289–293. doi: 10.1128/aac.5.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Smith B. L. Efficacy of diaminodiphenylsulfone and other drugs in murine Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1984 Oct;26(4):436–440. doi: 10.1128/aac.26.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D., Braveny I., Verhoef J. Clindamycin enhances opsonization of Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):413–417. doi: 10.1128/aac.24.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia B. A., Petito C. K., Gold J. W., Cho E. S., Jordan B. D., Price R. W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986 Mar;19(3):224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- Navin T. R., Miller K. D., Satriale R. F., Lobel H. O. Adverse reactions associated with pyrimethamine-sulfadoxine prophylaxis for Pneumocystis carinii infections in AIDS. Lancet. 1985 Jun 8;1(8441):1332–1332. doi: 10.1016/s0140-6736(85)92819-3. [DOI] [PubMed] [Google Scholar]
- Parkhurst G. W., Nora M. V., Thomas R. W., Carson P. E. High-performance liquid chromatographic-ultraviolet determination of primaquine and its metabolites in human plasma and urine. J Pharm Sci. 1984 Sep;73(9):1329–1331. doi: 10.1002/jps.2600730943. [DOI] [PubMed] [Google Scholar]
- Queener S. F., Bartlett M. S., Jay M. A., Durkin M. M., Smith J. W. Activity of lipid-soluble inhibitors of dihydrofolate reductase against Pneumocystis carinii in culture and in a rat model of infection. Antimicrob Agents Chemother. 1987 Sep;31(9):1323–1327. doi: 10.1128/aac.31.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Kelly J. A. Clindamycin-induced suppression of toxic-shock syndrome--associated exotoxin production. J Infect Dis. 1984 Mar;149(3):471–471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- Schmidt L. H. Enhancement of the curative activity of primaquine by concomitant administration of mirincamycin. Antimicrob Agents Chemother. 1985 Feb;27(2):151–157. doi: 10.1128/aac.27.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg L. S., Parquette A. R., Gluzman I. Y., Phillips G. W., Jr, Brodasky T. F., Krogstad D. J. Clindamycin activity against chloroquine-resistant Plasmodium falciparum. J Infect Dis. 1984 Dec;150(6):904–911. doi: 10.1093/infdis/150.6.904. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Bartlett M. S., Queener S. F., Durkin M. M., Jay M. A., Hull M. T., Klein R. S., Marr J. J. Pneumocystis carinii pneumonia therapy with 9-deazainosine in rats. Diagn Microbiol Infect Dis. 1987 Jun;7(2):113–118. doi: 10.1016/0732-8893(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Tuazon C. U., Labriola A. M. Management of infectious and immunological complications of acquired immunodeficiency syndrome (AIDS). Current and future prospects. Drugs. 1987 Jan;33(1):66–84. doi: 10.2165/00003495-198733010-00004. [DOI] [PubMed] [Google Scholar]
- White N. J. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985 May-Jun;10(3):187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- Wofsy C. B. Use of trimethoprim-sulfamethoxazole in the treatment of Pneumocystis carinii pneumonitis in patients with acquired immunodeficiency syndrome. Rev Infect Dis. 1987 Mar-Apr;9 (Suppl 2):S184–S194. doi: 10.1093/clinids/9.supplement_2.s184. [DOI] [PubMed] [Google Scholar]
- el Wakeel E. S., Homeida M. M., Ali H. M., Geary T. G., Jensen J. B. Clindamycin for the treatment of falciparum malaria in Sudan. Am J Trop Med Hyg. 1985 Nov;34(6):1065–1068. doi: 10.4269/ajtmh.1985.34.1065. [DOI] [PubMed] [Google Scholar]
- ul Haque A., Plattner S. B., Cook R. T., Hart M. N. Pneumocystis carinii. Taxonomy as viewed by electron microscopy. Am J Clin Pathol. 1987 Apr;87(4):504–510. doi: 10.1093/ajcp/87.4.504. [DOI] [PubMed] [Google Scholar]




