Abstract
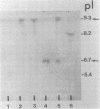
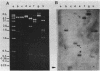
The L-1 penicillinase structural gene, blaS, from Pseudomonas maltophilia has been cloned into the vector pACYC184. The pMON01 recombinant plasmid selected by ampicillin resistance carried a 2.6-kilobase Sau3A fragment of P. maltophilia DNA and was confirmed to express L-1 beta-lactamase by comparative isoelectric focusing. A detailed physical map was constructed, and the blaS structural gene was localized with a 17-mer oligonucleotide mixed probe encoding the L-1 N-terminal amino acid sequence. Induction studies confirmed constitutive expression. Isolation of a complete beta-lactamase operon was attempted by construction of a P. maltophilia genomic library into phage lambda 2001. A recombinant phage was selected by DNA hybridization, and the 13.4-kilobase DNA insert was physically mapped and subcloned into plasmid vectors. Expression and L-1 beta-lactamase synthesis were studied in Escherichia coli.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Emanuel E. L., Gagnon J., Waley S. G. The production and molecular properties of the zinc beta-lactamase of Pseudomonas maltophilia IID 1275. Biochem J. 1985 Aug 1;229(3):791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush K., Sykes R. B. Methodology for the study of beta-lactamases. Antimicrob Agents Chemother. 1986 Jul;30(1):6–10. doi: 10.1128/aac.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas maltophilia infections in man. Am J Clin Pathol. 1969 Jan;51(1):58–61. doi: 10.1093/ajcp/51.1.58. [DOI] [PubMed] [Google Scholar]
- Grundström T., Jaurin B., Edlund T., Normark S. Physical mapping and expression of hybrid plasmids carrying chromosomal beta-lactamase genes of Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1127–1134. doi: 10.1128/jb.143.3.1127-1134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Tanaka T., Tsunekawa H., Aiba S. Cloning of the genes for penicillinase, penP and penI, of Bacillus licheniformis in some vector plasmids and their expression in Escherichia coli, Bacillus subtilis, and Bacillus licheniformis. J Bacteriol. 1981 Sep;147(3):776–786. doi: 10.1128/jb.147.3.776-786.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. Genetic regulation of penicillinase synthesis in Gram-positive bacteria. Microbiol Rev. 1978 Mar;42(1):67–83. doi: 10.1128/mr.42.1.67-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Grundström T., Normark S. Sequence elements determining ampC promoter strength in E. coli. EMBO J. 1982;1(7):875–881. doi: 10.1002/j.1460-2075.1982.tb01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J. S., Kahan F. M., Goegelman R., Currie S. A., Jackson M., Stapley E. O., Miller T. W., Miller A. K., Hendlin D., Mochales S. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 1979 Jan;32(1):1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Levesque R., Letarte R., Pechère J. C. Comparative study of the beta-lactamase activity found in Achromobacter. Can J Microbiol. 1983 Jul;29(7):819–826. doi: 10.1139/m83-133. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Muder R. R., Yu V. L., Dummer J. S., Vinson C., Lumish R. M. Infections caused by Pseudomonas maltophilia. Expanding clinical spectrum. Arch Intern Med. 1987 Sep;147(9):1672–1674. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]