Abstract
A major goal of cancer immunotherapy is the induction of a cell-mediated antitumor response in poorly immunogenic malignancies. We tested the hypothesis that this can be achieved by cytokine gene therapy with a novel histone H2A-based transient transfection procedure. This was tested by using cytokine genes encoding for IL-2 and a single chain IL-12 (scIL-12) fusion protein in a recently developed murine neuroblastoma model. Here, we demonstrate that cytokine gene transfer of IL-2 and scIL-12 with histone H2A results in the induction of an antitumor immune response that is superior in some respects to gene transfer with Superfect, a commercially available activated dendrimer commonly used to effect transfection with plasmids. Three lines of evidence support this contention. First, histone H2A-mediated transfection of IL-2 induces a natural killer cell-induced rejection of primary tumors in contrast to Superfect, which produces only a partial reduction in primary tumor growth. Second, the induction of a T cell-mediated protective tumor immunity following gene transfer of scIL-12 is more efficient with the histone H2A-mediated gene transfer because rejection of a lethal wild-type tumor cell challenge is accompanied by the greatest degree of MHC class I-restricted tumor cell killing in vitro. Third, histone H2A-mediated scIL-12 gene therapy induces the greatest release of mIFN-γ from splenocytes of vaccinated animals in contrast to Superfect and other controls.
The most challenging aspect of tumor vaccine development is the induction of an effective immune response against malignancies characterized by poor immunogenicity, e.g., neuroblastoma. One strategy to achieve this goal is the generation of autologous vaccines by isolation of the patient's tumor cells followed by their genetic engineering to secrete cytokines to enhance immunogenicity. Thus, a critical step in the development of such cellular vaccines is an efficient gene transfer that also translates into an optimal level of antitumor immunity.
The goal of cytokine antitumor gene therapy is to induce cellular immune responses against syngeneic malignancies by achieving cytokine concentrations in the tumor microenvironment sufficient to stimulate effector cells to elicit antitumor responses. The ex vivo modification of autologous tumor cells to express cytokines is currently under investigation in a variety of cancers (1). Once such cytokines are produced by tumor cells, they induce local inflammatory responses resulting in the elimination of transfected tumor cells and, in some cases, the induction of a systemic immune response effective against preexisting distant metastases (2). This strategy was also applied to neuroblastoma in preclinical animal models and initial clinical trials. Thus, ex vivo gene transfer of IL-2 in a murine neuroblastoma model decreased tumorigenicity and enhanced systemic immunity followed by the regression of preestablished hepatic metastases (3). IL-2 was established as a T cell growth factor and has remained the focus of considerable attention as an agent for cancer immunotherapy because of its stimulatory effect on a broad range of immune cell types, including both T and B cells, monocytes, macrophages, and natural killer (NK) cells. Based on these encouraging preclinical findings, autologous neuroblastoma cells isolated from ten stage 4 patients were transfected with IL-2 and used for s.c. vaccination. Six weeks postimmunization, one patient presented with a partial response, and four patients had stable disease. Subsequently, however, one of the patients with stable disease progressed to a complete tumor response (4). An alternative cytokine that has increasingly been used for cytokine gene therapy is IL-12, based on its ability to activate naïve T cells, thereby facilitating T cell priming in contrast to IL-2, which primarily amplifies and proliferates primed T cells. Because of the occurrence of fatal toxicity in a clinical trial, investigators focused on local administration of this cytokine by gene therapy. Gene therapy with heterodimeric IL-12 involves the delivery of equimolar amounts of both p35 and p40 subunits, thus eliminating overexpression of p40, which is associated with the formation of inhibitory IL-12 p40 dimers. This problem was resolved by the genetic linkage of p35 and p40 using a DNA sequence encoding for a flexible protein linker commonly used in antibody engineering. This single chain (sc) IL-12 construct demonstrated efficacy for gene therapy in a neuroblastoma model as demonstrated by the induction of a T cell-mediated immunity that completely protected mice from lethal challenge with wild-type tumor cells (5). These experiments involved the use of stably transfected neuroblastoma cells as cellular vaccines; however, such cells are nearly impossible to generate from freshly isolated tumor cells from patients. Therefore, effective transient gene delivery systems that lead to optimal immunological responses are needed.
In this study, we demonstrate that gene delivery of cytokine genes using a novel histone H2A gene delivery system results in an increase in some parameters in the antitumor immune response when compared with an activated dendrimer. This was demonstrated for both IL-2 and scIL-12 genes in a relevant preclinical model of murine neuroblastoma. Furthermore, the immune mechanisms involved in the antitumor immune response revealed that induction of T cell-mediated protective immunity occurred only with scIL-12 and not IL-2.
Materials and Methods
Cells and Experimental Metastasis Model of Neuroblastoma.
NXS2 neuroblastoma cells were grown in DMEM supplemented with 10% FCS (HyClone) as previously reported (6). Syngeneic female A/J mice were obtained at 6–8 weeks of age from The Jackson Laboratory or from the breeding colony at The Scripps Research Institute. Animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental liver and bone marrow metastases were induced by i.v. injection of 5 × 104 NXS2 cells and analyzed as previously described (7).
Cytokine Gene Transfer.
Coding sequences for mouse IL-2 and scIL-12 were cloned into the expression vector pcDNA3.1 (Invitrogen) as previously described (5, 8) and produced in large quantities using DH5α cells (Life Technologies Grand Island, NY) followed by plasmid preparation with Endofree plasmid kits (Qiagen, Chatsworth, CA). Supercoiled DNA was quantitated on 0.9% agarose gels using the Eagle Eye System (Stratagene).
To optimize cytokine secretion by NXS2 cells following IL-2 and scIL-12 gene transfer, varying plasmid DNA and histone H2A or Superfect concentrations were tested. For this purpose, NXS2 cells were plated at a density of 8 × 104 cells per well in a 96-well plate and transfected the following day with varying concentrations of plasmid DNA and histone H2A (Boehringer Mannheim, Germany) or Superfect (Qiagen). Production of IL-2 and scIL-12 was quantified 24 h later by ELISA assays (BioSource International, Camarillo, CA).
The preparation of large amounts of cytokine-secreting NXS2 cells for subsequent immunization experiments was achieved in 6-well plates. Thus, 2.4 × 106 NXS2 cells were plated per well and transfected the following day. Histone H2A (Boehringer Mannheim) was diluted in LAL Reagent Water (BioWhittaker) and the expression vectors in Tris-acetate buffer (pH 8.0) to a final concentration of 240 mM. In each well of a 6-well plate, 0.6 ml of histone H2A (Boehringer Mannheim) was combined with 0.6 ml of plasmid DNA at room temperature for 30 min. Then, 1.2 ml of OptiMEM (Life Technologies) medium was added to the histone–DNA mixture. The media of the overnight cultures were removed and replaced with the 2.4-ml mixture of histone–DNA complexes and OptiMEM. Four hours later, 1.2 ml of OptiMEM (30% FCS) was added to each well followed by a medium change after 24 h with OptiMEM (10% FCS).
In the Superfect group, 0.1 ml of DNA (240 mM Tris-acetate buffer, pH 8.0) was combined at room temperature with 8 μl of Superfect for 10 min followed by the addition of 0.4 ml of DMEM. The medium of the overnight cultures was removed and replaced with a 0.5-ml mixture of Superfect-DNA. Three hours later, the medium was replaced with 3.6 ml of OptiMEM (10% FCS) followed by a change of medium 24 h later with OptiMEM (10% FCS). The medium was removed 48 h after the start of transfection and stored at −70°C for subsequent cytokine ELISA assays. Cells harvested by trypsinization were checked for viability by trypan blue staining and prepared for s.c. injection in unsupplemented DMEM, only if viability exceeded 95%.
Immunization Experiments.
Mice were immunized by s.c. injection with 2 × 106 NXS2 cells genetically engineered to secrete IL-2 or scIL-12 and compared with empty vector and naïve mice controls. Primary tumor growth was determined over time by microcaliper measurements and the size calculated according to ½ × width2 × length. Subsequently, mice were challenged by i.v. injection with 5 × 104 wild-type NXS2 cells 7 or 14 days after initial vaccination, and experimental metastases were documented. Depletion of immune effector cells was done by i.p. injections of anti-asialo GM1 antiserum or anti-CD4 or anti-CD8 monoclonal antibodies as previously described (6).
Cytotoxicity Assay and IFN-γ Release.
Splenocytes of vaccinated mice were isolated and cultured for 5 days in the presence of irradiated NXS2 cells in T cell medium [DMEM, 10% FCS, 2 mM L-glutamine, 5 × 10-4 M β-mercaptoethanol, penicillin (100 units/ml), streptomycin (100 μg/ml), and 1:25 T-STIM, (BDB Bioscience, Bedford, MA)] and then used in a standard 4-h chromium release assay against NXS2 target cells (6). Major histocompatibility complex (MHC) class I restriction was determined in the presence of 25 μg/ml anti-H2Kk MHC class I antibody (clone 36-7-5; PharMingen). The production of IFN-γ was measured in cultures of splenocytes by daily sampling of 100 μl of supernatant and subsequent analysis in a sandwich ELISA. For this purpose, capture antibody (clone 37895.11) and biotinylated detection antibody (clone 37801.11) were purchased from R&D Systems and used according to the manufacturer's protocol. Recombinant mIFN-γ standard (PharMingen) was used for quantification.
Statistical Analysis.
The statistical significance of differential findings between experimental groups was determined by Student's t test. The nonparametric Wilcoxon test was used to determine the statistical significance of metastatic scores. Findings were regarded as significant if two-tailed P values were <0.05.
Results
Local Antitumor Response After Transient Production of IL-2 and scIL-12.
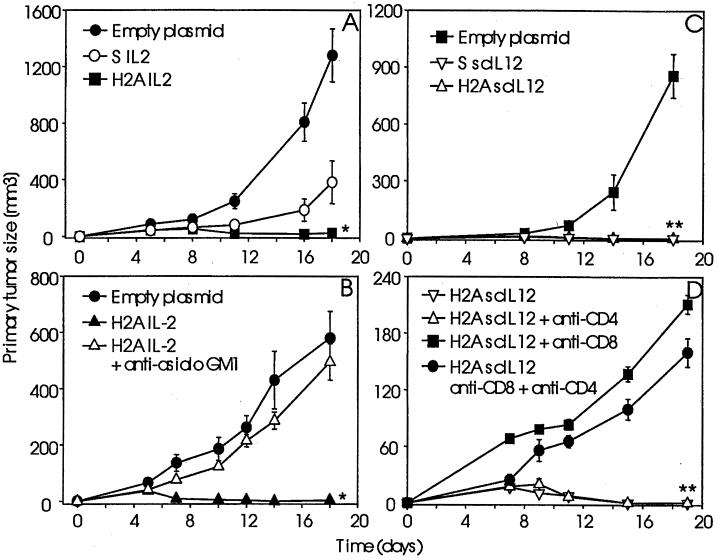
The evaluation of the antitumor immune response of syngeneic A/J mice against IL-2- and scIL-12-producing NXS2 cells was established following transient cytokine gene transfer with H2A or Superfect. For this purpose, IL-2 and scIL-12 producing NXS2 cells were administered by s.c. injection, and primary tumor growth was determined over time and compared with empty vector controls (Fig. 1). NXS2 cells transfected with IL-2 or scIL-12 using H2A were completely rejected. This was in contrast to IL-2 gene transfer by Superfect and empty vector controls that resulted in continuous s.c. tumor growth (Fig. 1A).
Figure 1.
Antitumor immune response following histone H2A-mediated cytokine gene transfer. The antitumor immune response was determined against s.c. injections with 2 × 106 NXS2 cells genetically engineered to transiently produce IL-2 (A and B) and scIL-12 (C and D) using histone H2A. Syngeneic A/J mice (n = 6) were injected with NXS2 cells transfected with the IL-2 gene using H2A and compared with Superfect (S) and empty plasmid controls (A). Antitumor responses against IL-2 producing NXS2 cells following histone H2A-mediated IL-2 gene transfer were analyzed in A/J mice depleted of NK cells using anti-asialo GM1 (n = 4) and compared with non-depleted (n = 6) and empty plasmid controls (n = 6) (B). IL-12 gene transfer-mediated antitumor immune responses were analyzed in syngeneic A/J mice (n = 6) (C) and in A/J mice depleted of CD8+ and/or CD4+ T cells (D). Primary tumor size was determined by microcaliper measurements and calculated according to ½ × width2 × length. Each data point represents the mean value ± SE. The differences between experimental groups and control groups were statistically significant (*, P < 0.01, **, P < 0.001).
The determination of the immune mechanisms involved in primary tumor rejection following IL-2 and scIL-12 gene transfer revealed a clear dichotomy in immune effector cells eliciting primary tumor rejection, depending on the nature of the cytokine. This contention is based on the observation that depletion of NK cells with anti-asialo-GM1 antiserum resulted in the complete abrogation of the antitumor effect by local IL-2 production (Fig. 1B). This is in contrast to results obtained with local scIL-12 production. Here, depletion of CD8+ T cells, either alone or in combination with additional CD4+ T cell depletion, abrogated rejection of scIL-12-producing NXS2 cells in contrast to depletion of CD4+ T cells alone, which was ineffective in this regard (Fig. 1D).
Effect of Transient IL-2 and scIL-12 Gene Delivery on Systemic Antitumor Immunity.
The induction of a systemic protective immunity following s.c. injection of genetically engineered NXS2 cells that produced IL-2 and scIL-12 was tested by a subsequent i.v. challenge with wild-type NXS2 cells, 7 and 14 days after initial s.c. inoculation. Protective immunity was determined by the evaluation of liver and bone marrow metastases 28 days after challenge in immunized animals and compared with controls such as naïve mice and mice that received NXS2 cells transfected with the empty vector. Only mice receiving s.c. injections of scIL-12-producing NXS2 cells presented with a complete absence of liver and bone marrow metastases in the majority of animals, consistent with the development of an immunization effect (Table 1). Interestingly, this was in contrast to similar experiments using IL-2. Thus, mice injected with NXS2 cells producing IL-2 were not protected against wild-type NXS2 cell challenge as indicated by high levels of liver and bone marrow metastases identical to that of naïve mice or mice injected with NXS 2 cells transfected with the empty vector. These data clearly indicate the superior activity of scIL-12 in the induction of a vaccination effect in the neuroblastoma model.
Table 1.
Effect of transient secretion of IL-2 and scIL-12 by NXS2 neuroblastoma cells on the induction of a systemic protective immunity
| Vaccine* | Day of challenge | Bone marrow metastasis† | Liver‡ metastasis | Liver weight,§ mg |
|---|---|---|---|---|
| None | 14 | 2,2,2,2,2,2 | 4,4,4,4,4,4 | 3950 ± 310 |
| NXS2 S empty plasmid | 14 | 2,2,2,2,1,1 | 4,4,4,2,2,1 | 2558 ± 425 |
| NXS2 S IL2 | 14 | 2,2,2,2,2,1 | 4,4,4,4,3,1 | 3600 ± 673 |
| NXS2 H2A IL2 | 14 | 2,2,2,2 | 4,4,4,4 | 4337 ± 452 |
| None | 7 | 2,2,2,1 | 4,4,4,4 | 3466 ± 115 |
| NXS2 S empty plasmid | 7 | 2,2,2 | 4,4,4 | 3465 ± 205 |
| NXS2 H2A IL2 | 7 | 2,2,2,2,2,2 | 4,4,4,4,4,2 | 3032 ± 215 |
| NXS2 H2A empty plasmid | 7 | 2,2,2,2,1,1 | 4,4,4,4,3,2 | 3053 ± 265 |
| NXS2 S empty plasmid | 7 | 2,2,2,1,1,1 | 4,4,4,3,2,1 | 2043 ± 201 |
| NXS2 S scIL-12¶ | 7 | 0,0,0,0,0,1 | 0,0,0,0,1,2 | 1290 ± 74 |
| NXS2 H2A scIL-12¶ | 7 | 0,0,0,0,1,1 | 0,0,0,0,1,2 | 1312 ± 31 |
Mice were injected s.c. with 2 × 106 NXS2 cells genetically engineered to produce IL-2 and scIL-12 and challenged by a lethal intravenous injection of 5 × 104 NXS2 wild-type cells 7 and 14 days after initial inoculation.
NXS2 cells were transfected with Superfect (S) or histone H2A (H2A) using IL-2 and scIL-12 plasmid DNA and compared to empty plasmid controls.
Bone marrow metastases were staged according to results obtained by high and low sensitivity tyrosine hydroxylase RT-PCR as described in Materials and Methods.
Liver metastases were staged according to the percentage of metastatic liver surface: 0, 0%; 1, <0–25%; 2, 25–50%; 3, 50–75%; 4, >75%.
Liver weight was determined on fresh specimens and expressed as mean values ± SE.
Differences in bone marrow staging, liver metastasis, and liver weights between experimental groups and control groups were statistically significant (P < 0.01).
The determination of the type of effector cells involved in mediating protective immunity following H2A-mediated scIL-12 gene delivery was accomplished by in vivo depletion of CD8+ and/or CD4+ T cells. In fact, only vaccinated animals depleted of CD8+ T cells, in the presence or absence of additionally depleted CD4+ T cells, revealed a complete abrogation of protective immunity (Table 2), indicating a CD8+ T cell-mediated mechanism. The depletion of CD4+ T cells induced partial abrogation of tumor protective immunity in 2 of 4 mice, suggesting a helper function of the CD4+ T cell subpopulation in the protective immunity induced by scIL-12 gene therapy.
Table 2.
Effect of T cell depletion on induction of systemic protective immunity by NXS2 neuroblastoma cells transiently secreting scIL-12
| Vaccine | Depletion* | Bone marrow† metastasis | Liver‡ metastasis | Liver weight,§ mg |
|---|---|---|---|---|
| None | None | 2,2,2,2 | 4,4,4,4 | 3112 ± 105 |
| NXS2 S empty pl. | None | 2,2,2,1,1,0 | 4,3,2,1,1,1 | 2465 ± 137 |
| NXS2 H2A scIL12 | None | 0,0,0,0,0,1 | 0,0,0,0,0,1 | 1063 ± 29 |
| NXS2 H2A scIL12 | CD4 | 1,1,0,0 | 3,3,0,0 | 1510 ± 124 |
| NXS2 H2A scIL12¶ | CD8 | 2,2,1,1 | 4,4,3,2 | 2408 ± 299 |
| NXS2 H2A scIL12¶ | CD4 + CD8 | 2,2,2,1 | 4,4,2,1 | 2395 ± 436 |
Mice were injected s.c. with 2 × 106 NXS2 cells genetically engineered to secrete scIL-12 using histone H2A (H2A) and challenged by a lethal intravenous injection of 5 × 104 NXS2 wild-type cells 7 days after initial inoculation.
T cells were depleted by i.p. injection of 500 μg of anti-CD4 and anti-CD8 antibodies at days 0, 7, 14, and 21.
Bone marrow metastases were staged according to results obtained by high and low sensitivity tyrosine hydroxylase RT-PCR as described in Materials and Methods.
Liver metastases were staged according to the percentage of metastatic liver surface: 0, 0%; 1, <0–25%; 2, 25–50%; 3, 50–75%; 4, >75%.
Liver weight was determined on fresh specimen and expressed as mean values ± SE.
Differences in bone marrow staging, liver metastasis, and liver weights between CD8-depleted groups and the nondepleted control group were statistically significant (P < 0.05).
Amplitude of Cytotoxic T Lymphocyte (CTL) Response and mIFN-γ Release Following H2A- and Superfect-Mediated Cytokine Gene Transfer.
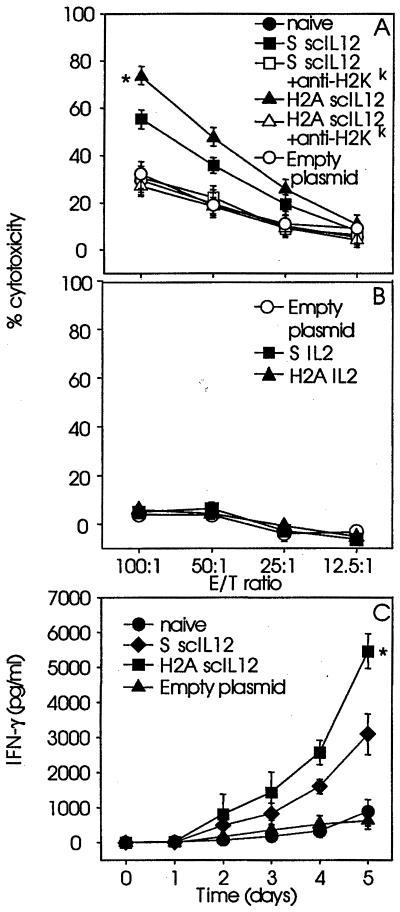
Further characterization of the immune response by determination of CTL activity and mIFN-γ release revealed a greater efficacy of H2A-mediated than of Superfect-mediated scIL-12 gene transfer. This was indicated by increased CTL activity and mIFN-γ release observed in splenocyte cultures generated from mice vaccinated with NXS2 cells genetically engineered to secrete scIL-12 by transfection mediated by H2A when compared with Superfect (Fig. 2). The cytotoxic activity elicited by splenocytes was inhibited in the presence of anti-MHC class I antibodies (Fig. 2A), a classical feature of cytolytic CD8+ T cell (CTL)-mediated antitumor responses. The presence of CTLs following vaccination with NXS2 cells engineered to secrete scIL-12 (Fig. 2A) is consistent with the protective immunity directed against wild-type tumor cell challenge observed in this system (Tables 1 and 2). No CTL response was detected in similar experiments with IL-2-secreting NXS2 cells (Fig. 2B), a finding that is also consistent with the absence of protective immunity following wild-type tumor cell challenge (Table 1).
Figure 2.
Effect of histone H2A-mediated cytokine gene transfer on tumor cytolysis and IFN-γ production. Splenocytes of mice (n = 3) injected with 2 × 106 NXS2 cells genetically engineered to transiently produce scIL-12 (A and C) and IL-2 (B) were isolated 14 days after NXS2 cell injection and used in a standard 51Cr release assay against NXS2 target cells (A and B) or analyzed for the production of IFN-γ by ELISA (C). Each data point represents mean value ± SE. Differences between experimental groups and control groups were statistically significant (P < 0.005).
Discussion
The induction of effective antitumor immune responses by cytokine gene delivery is an important strategy for cancer immunotherapy. This requires a simple, yet rapid and efficacious means to achieve gene expression that is readily accomplished by transient transfection. In contrast, the more laborious and time-consuming methodology of stable gene transfer requiring established autologous tumor cell lines from each individual patient is nearly impossible to realize.
Here, we demonstrate that transient transfection using a histone H2A-mediated gene delivery system of IL-2 and scIL-12 cytokine genes efficiently induces an antitumor immune response in a relevant model of murine neuroblastoma. Interestingly, tumor protection by transient scIL-12 gene transfer was similar to that achieved by stable transfection of scIL-12 into NXS2 cells, demonstrating equivalent efficiency of transient scIL-12 gene delivery using histone H2A versus the more tedious stable transfection procedures. Gene transfer using histone H2A has been found to depend on distinct molecular characteristics and is not merely attributable to the presence of a positive charge; transfections of COS-7 cells with a β-galactosidase reporter plasmid using poly-L-lysine, poly-L-arginine, and a mixture of poly-L-lysine and poly-L-arginine (9) or polybrene, spermine, and spermidine (data not shown) at equimolar ratios as represented in histone H2A were all ineffective. Interestingly, all of the cationic polymers tested bound to DNA, as demonstrated by agarose gel electrophoresis (data not shown). These results suggest that DNA binding alone is insufficient for transfection and that a nuclear localization signal of histone H2A might be responsible for its remarkable capacity to efficiently mediate gene delivery. We found that in a number of ways, the response to histone H2A was more efficient than that of Superfect.
Histone H2A-mediated transfection of IL-2 induces an NK cell-mediated rejection of primary tumors in contrast to Superfect, which produced only a 60% reduction in primary tumor growth. The induction of T cell-mediated protective tumor immunity following gene transfer of scIL-12 is more efficient with histone H2A-mediated gene transfer than with Superfect, as the rejection of a lethal wild-type tumor cell challenge is accompanied by the highest degree of MHC class I-restricted tumor cell killing in vitro. In addition, histone H2A-mediated scIL-12 gene therapy induced a greater release of mIFN-γ from splenocytes of vaccinated animals than did Superfect. The reason for this apparent advantage of histone H2A over Superfect is not clear. We do not know to what extent these in vitro findings are mirrored by the in vivo secretion of cytokines by tumor cells transfected by histone H2A or Superfect.
The characteristics of the immune response following H2A-mediated scIL-12 and IL-2 gene therapy differed for the two cytokines. Specifically, scIL-12 was able to induce a CD8+ T cell-mediated protective immune response in contrast to IL-2, which was ineffective in this respect. This finding is consistent with observations made with a tumor-specific antibody–IL-2 fusion protein (IL-2 immunocytokine) recognizing disialoganglioside GD2 overexpressed in >95% of human neuroblastoma cells, as well as in this NXS2 neuroblastoma model (7, 10). The concept of tumor-specific targeting of IL-2 by such a construct was demonstrated in the NXS2 neuroblastoma model, where it led to a local increase of IL-2 in the tumor microenvironment, similar to the cytokine gene therapy outlined in this report. Treatment with IL-2 immunocytokine resulted in the eradication of liver and bone marrow metastases, indicating an effective in vivo antitumor immune response. Analogous to the IL-2 gene therapy outlined in this report, the previously published immune mechanism was exclusively mediated by NK cells (6). The absence of a T cell-mediated mechanism was not related to peak levels of IL-2 in the tumor microenvironment, as lowering the dose of IL-2 resulted in loss of immunotherapeutic affects without any CTL priming. The NK cell-mediated immune response was established by in vivo depletion experiments that revealed that treatment effects were only abrogated in mice depleted of NK cells in contrast to CD8+ T cell-depleted animals where IL-2 immunocytokine therapy remained completely effective. Furthermore, successfully treated mice did not develop an immune protection against challenges with wild-type NXS2 cells, an observation similar to that obtained with IL-2 gene therapy (Table 1). Based on these findings, it seems that IL-2 is of limited value for the initial induction of a CD8+ T cell response in this neuroblastoma system, an observation that is entirely in contrast to scIL-12 therapy, which proved effective in this regard in two independent experimental settings, i.e., transient gene delivery and stable transfection of NXS2 neuroblastoma cells. Interestingly, initial clinical trials of IL-2 gene therapy with autologous tumor cells transfected with IL-2 by adenoviral gene delivery also revealed only limited efficacy in induction of a CD8+ T cell immune response, a finding that is in agreement with observations made in the neuroblastoma model. Therefore, the results obtained with scIL-12 in this system as reported earlier and outlined in this study suggest that scIL-12 might be of greater value for active immunotherapy of this disease than IL-2.
Acknowledgments
This work was supported by the Medical Research Council of Canada Clinician Scientist Award (to D.B.), National Institutes of Health Grant CA42508 (to R.A.R.), and the Stein Endowment Fund (to E.B.). This is manuscript 13368-MEM from The Scripps Research Institute.
Abbreviations
- NK
natural killer
- sc
single chain
- CTL
cytotoxic T lymphocyte
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210382997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210382997
References
- 1.Roth J A, Cristiano R J. J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll D M. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H, Tanabe M, Miyauchi M, Kawamura K, Takenaga K, Ohnuma N, Sakiyama S, Tagawa M. Cancer Gene Ther. 1999;6:395–401. doi: 10.1038/sj.cgt.7700052. [DOI] [PubMed] [Google Scholar]
- 4.Bowman L, Grossmann M, Rill D, Brown M, Zhong W Y, Alexander B, Leimig T, Coustan-Smith E, Campana D, Jenkins J, et al. Blood. 1998;92:1941–1949. [PubMed] [Google Scholar]
- 5.Lode H N, Dreier T, Xiang R, Varki N M, Kang A S, Reisfeld R A. Proc Natl Acad Sci USA. 1998;95:2475–2480. doi: 10.1073/pnas.95.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lode H N, Xiang R, Dreier T, Varki N M, Gillies S D, Reisfeld R A. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 7.Lode H N, Xiang R, Varki N M, Dolman C S, Gillies S D, Reisfeld R A. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 8.Dreier T, Lode H N, Xiang R, Dolman C S, Reisfeld R A, Kang A S. Bioconjugate Chem. 1998;9:482–489. doi: 10.1021/bc980020e. [DOI] [PubMed] [Google Scholar]
- 9.Balicki D, Beutler E. Mol Med. 1997;3:782–787. [PMC free article] [PubMed] [Google Scholar]
- 10.Brodeur G M, Pritchard J, Berthold F, Carlsen N L, Castel V, Castelberry R P, De Bernardi B, Evans A E, Favrot M, Hedborg F. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]