Abstract
AIM—To investigate an image distortion, experienced by patients with homonymous paracentral scotomas. METHODS—Two consecutive patients with right inferior homonymous paracentral scotomas resulting from ischaemic brain insults were examined. Neuro-ophthalmological examination included tangent screen and Amsler grid evaluation. In addition, the patients were asked to describe a figure showing two vertical lines, identical in length and symmetrically located on either side of a fixation point. This figure was presented in such a way that when the subject looked at the fixation point the right line crossed the scotoma. Finally, the patients were asked whether, when looking at the face of an interlocutor, both sides of the body looked the same. RESULTS—In both patients field defects were markedly smaller when delineated with Amsler grids than using a tangent screen. With the parallel line test, the right line appeared uninterrupted in patient 1, whereas in patient 2 it looked slightly blurred in a two degree long segment corresponding to the middle of the scotoma. To both subjects the right line appeared shorter than the left line. Finally, both subjects indicated that, after steadily fixating their interlocutor's face or neck for 5-10 seconds, the left shoulder appeared narrower than the right one, which made him look surprisingly thin. This perceptual alteration was called the "thin man" phenomenon. CONCLUSIONS—Paracentral homonymous scotomas can be associated with perceptual completion and shape distortion, owing to apparent displacement of images adjacent to the scotoma towards the field defect. Occurrence of such a perceptual change should alert one to the possibility of paracentral homonymous scotomas, which often go undetected when using routine visual field testing procedures. Keywords: paracentral scotomas; cortical plasticity; "thin man" phenomenon
Full Text
The Full Text of this article is available as a PDF (112.9 KB).
Figure 1 .
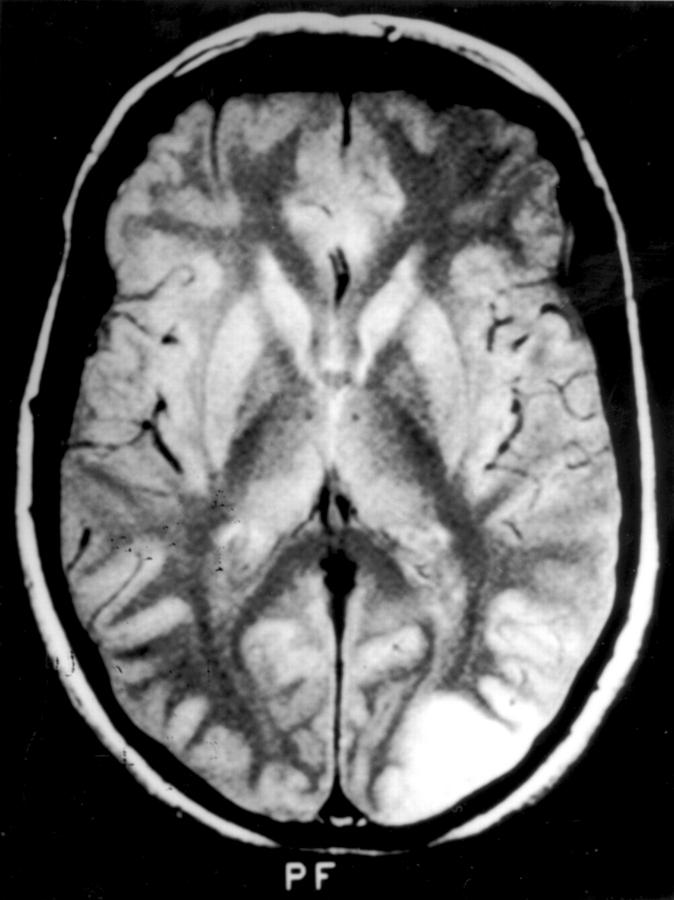
Cerebral magnetic resonance image of patient 1, showing, on a T2 weighted image, a left sided lesion extending from the occipital pole into the lateral occipital gyri.
Figure 2 .
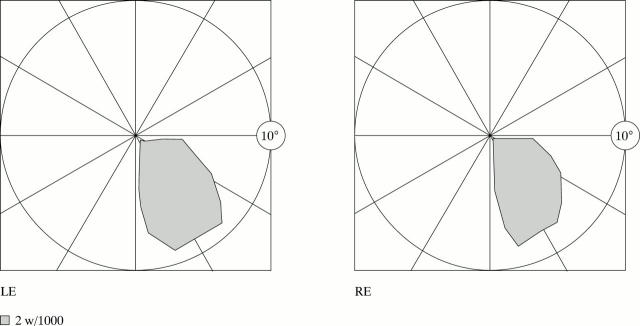
Right paracentral homonymous scotoma of patient 1, delineated by tangent screen.
Figure 3 .
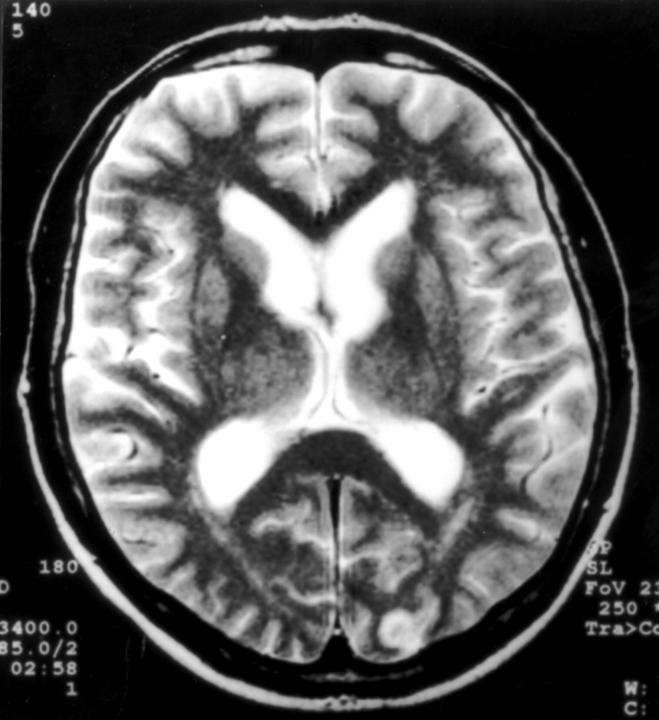
Cerebral magnetic resonance image of patient 2, showing, on a T2 weighted image, a single small lesion at the left occipital pole.
Figure 4 .
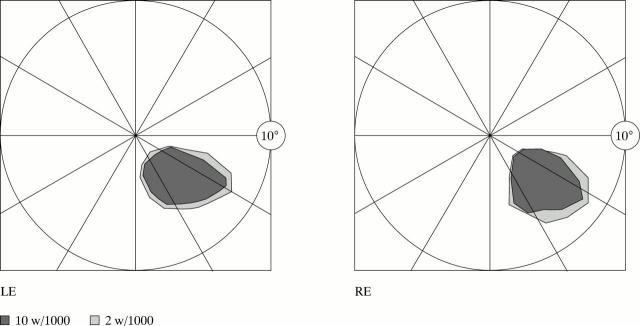
Right paracentral homonymous scotomas of patient 2, delineated by tangent screen.
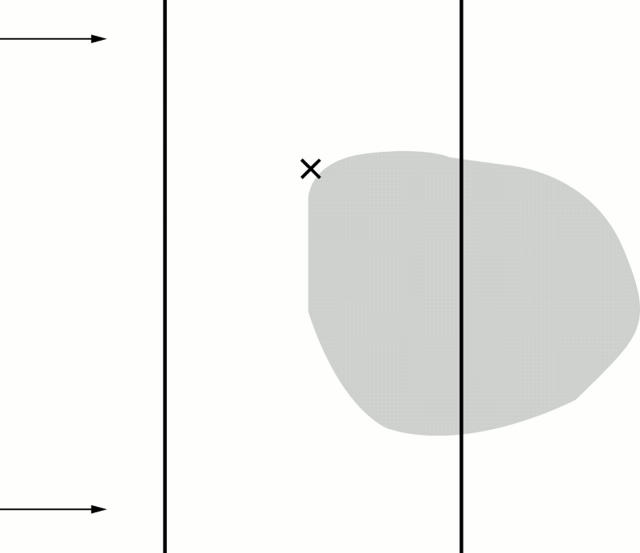
Figure 5 .
Schematic representation of the parallel line test evaluating the completion phenomenon in subjects with a paracentral scotoma. It shows the relative positions of the fixation point (cross), vertical test lines, and right paracentral scotoma (shaded area). The vertical lines are equal in length and are equidistant from the fixation point. They are presented to the subject in such a way that the line located on the affected side of the visual field crosses the scotoma, and extends for a short distance on either side of it. After steadily fixating the cross for 5-10 seconds, the subject is asked about any perceived interruption in the lines or inequality in their length, and is asked to indicate, on the line located on the unaffected side, the levels corresponding to the perceived upper and lower ends of line on the affected side (as exemplified by the two arrows).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achard O. A., Safran A. B., Duret F. C., Ragama E. Role of the completion phenomenon in the evaluation of Amsler grid results. Am J Ophthalmol. 1995 Sep;120(3):322–329. doi: 10.1016/s0002-9394(14)72162-2. [DOI] [PubMed] [Google Scholar]
- Brust J. C., Behrens M. M. "Release hallucinations" as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977 Nov;2(5):432–436. doi: 10.1002/ana.410020516. [DOI] [PubMed] [Google Scholar]
- Cohen L., Gray F., Meyrignac C., Dehaene S., Degos J. D. Selective deficit of visual size perception: two cases of hemimicropsia. J Neurol Neurosurg Psychiatry. 1994 Jan;57(1):73–78. doi: 10.1136/jnnp.57.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Gilbert C. D. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995 Jun 29;375(6534):780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- De Weerd P., Gattass R., Desimone R., Ungerleider L. G. Responses of cells in monkey visual cortex during perceptual filling-in of an artificial scotoma. Nature. 1995 Oct 26;377(6551):731–734. doi: 10.1038/377731a0. [DOI] [PubMed] [Google Scholar]
- Desimone R., Schein S. J. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J Neurophysiol. 1987 Mar;57(3):835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Ebata S., Ogawa M., Tanaka Y., Mizuno Y., Yoshida M. Apparent reduction in the size of one side of the face associated with a small retrosplenial haemorrhage. J Neurol Neurosurg Psychiatry. 1991 Jan;54(1):68–70. doi: 10.1136/jnnp.54.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Plasticity in visual perception and physiology. Curr Opin Neurobiol. 1996 Apr;6(2):269–274. doi: 10.1016/s0959-4388(96)80083-3. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Kapadia M. K., Gilbert C. D., Westheimer G. A quantitative measure for short-term cortical plasticity in human vision. J Neurosci. 1994 Jan;14(1):451–457. doi: 10.1523/JNEUROSCI.14-01-00451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb M., Petersen D., Schiefer U., Kolb R., Skalej M. Scotoma perception in white-noise-field campimetry and postchiasmal visual pathway lesions. Ger J Ophthalmol. 1995 Jul;4(4):228–233. [PubMed] [Google Scholar]
- MOONEY A. J., CAREY P., RYAN M., BOFIN P. PARASAGITTAL PARIETO-OCCIPITAL MENINGIOMA WITH VISUAL HALLUCINATIONS. Am J Ophthalmol. 1965 Feb;59:197–205. [PubMed] [Google Scholar]
- MULLAN S., PENFIELD W. Illusions of comparative interpretation and emotion; production by epileptic discharge and by electrical stimulation in the temporal cortex. AMA Arch Neurol Psychiatry. 1959 Mar;81(3):269–284. [PubMed] [Google Scholar]
- Pettet M. W., Gilbert C. D. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V. S. Blind spots. Sci Am. 1992 May;266(5):86–91. doi: 10.1038/scientificamerican0592-86. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991 Apr 25;350(6320):699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Safran A. B., Landis T. Plasticity in the adult visual cortex: implications for the diagnosis of visual field defects and visual rehabilitation. Curr Opin Ophthalmol. 1996 Dec;7(6):53–64. doi: 10.1097/00055735-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Lee K. The role of the primate extrastriate area V4 in vision. Science. 1991 Mar 8;251(4998):1251–1253. doi: 10.1126/science.2006413. [DOI] [PubMed] [Google Scholar]