Abstract
AIMS—To demonstrate the quantitative distribution of mitochondrial enzymes within the human optic nerve and retina in relation to the pathogenesis of ophthalmic disease. METHODS—Enucleations were performed at the time of multiple organ donation and the optic nerve and peripapillary retina immediately excised en bloc and frozen. Reactivities of the mitochondrial enzymes cytochrome c oxidase and succinate dehydrogenase were demonstrated in serial cryostat sections using specific histochemical assays. RESULTS—In the optic nerve the unmyelinated prelaminar and laminar regions were rich in both cytochrome c oxidase and succinate dehydrogenase. Myelination of fibres as they exited the lamina cribrosa was associated with an abrupt reduction in enzyme activity. Within the retina, high levels of enzyme activity were found localised within the retinal ganglion cells and nerve fibre layer, the outer plexiform layer, inner segments of photoreceptors, and the retinal pigment epithelium. CONCLUSIONS—Mitochondrial enzyme activity is preserved in human optic nerve and retina retrieved at the time of multiple organ donation. The distribution of enzyme activity within the eye has implications for the understanding of the pattern of ophthalmic involvement seen in mitochondrial diseases and the site of ganglion cell dysfunction in those patients with optic nerve involvement. Keywords: mitochondria; optic nerve; retina
Full Text
The Full Text of this article is available as a PDF (174.2 KB).
Figure 1 .
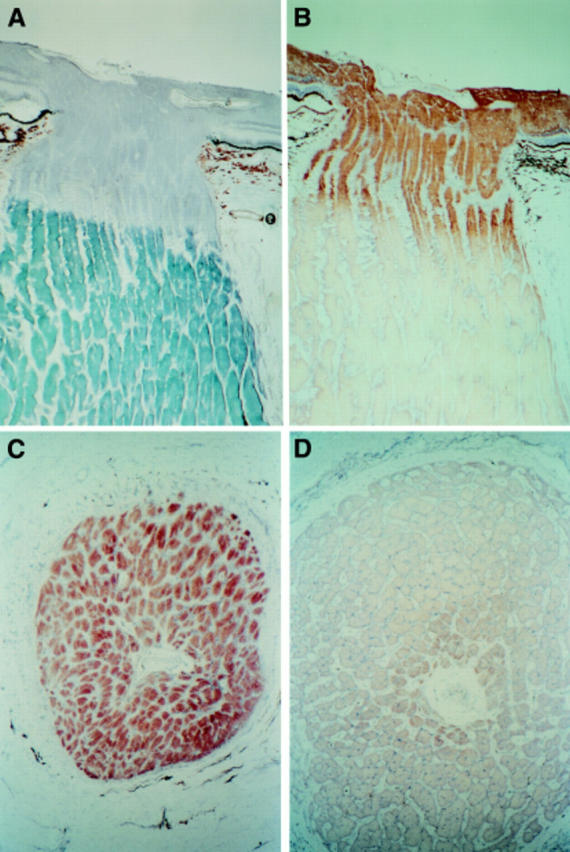
Photomicrographs of longitudinal sections through the optic nerve (A) stained with Sudan black to demonstate myelin and (B) reacted to demonstrate COX which is confined to the unmyelinated portion of retinal ganglion cell axons. Transverse sections of optic nerve (C) in immediate prelaminar zone showing high COX activity and (D) in the retrolaminar zone approximately 1.5 mm from section (C) showing low COX activity.
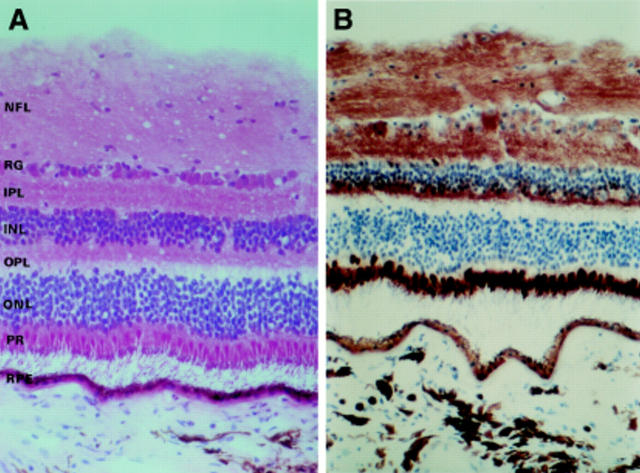
Figure 2 .
Photomicrographs of longitudinal sections of peripapillary retina (A) stained using haematoxylin and eosin, and (B)showing the distribution and intensity of COX activity in the different retinal layers. See text for abbreviations.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992 May 15;284(Pt 1):1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., MacDonald I., Wallace D. C. Leber's hereditary optic neuropathy: a model for mitochondrial neurodegenerative diseases. FASEB J. 1992 Jul;6(10):2791–2799. doi: 10.1096/fasebj.6.10.1634041. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Silver I. A. ATP and brain function. J Cereb Blood Flow Metab. 1989 Feb;9(1):2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Howell N. Leber hereditary optic neuropathy: how do mitochondrial DNA mutations cause degeneration of the optic nerve? J Bioenerg Biomembr. 1997 Apr;29(2):165–173. doi: 10.1023/a:1022690030664. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Kadenbach B., Droste M., Old S. L., Turnbull D. M. Immunocytochemical studies of cytochrome oxidase subunits in skeletal muscle of patients with partial cytochrome oxidase deficiencies. J Neurol Sci. 1988 Oct;87(1):75–90. doi: 10.1016/0022-510x(88)90056-1. [DOI] [PubMed] [Google Scholar]
- Kadekaro M., Crane A. M., Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama G. H., Wong-Riley M. T. The histochemical localization of cytochrome oxidase in the retina and lateral geniculate nucleus of the ferret, cat, and monkey, with particular reference to retinal mosaics and ON/OFF-center visual channels. J Neurosci. 1984 Oct;4(10):2445–2459. doi: 10.1523/JNEUROSCI.04-10-02445.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama G. H., Wong-Riley M. Laminar and cellular localization of cytochrome oxidase in the cat striate cortex. J Comp Neurol. 1986 Mar 8;245(2):137–159. doi: 10.1002/cne.902450202. [DOI] [PubMed] [Google Scholar]
- Lessell S., Horovitz B. Histochemical study of enzymes of optic nerve of monkey and rat. Am J Ophthalmol. 1972 Jul;74(1):118–126. doi: 10.1016/0002-9394(72)91135-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Old S. L., Johnson M. A. Methods of microphotometric assay of succinate dehydrogenase and cytochrome c oxidase activities for use on human skeletal muscle. Histochem J. 1989 Sep-Oct;21(9-10):545–555. doi: 10.1007/BF01753355. [DOI] [PubMed] [Google Scholar]
- Riordan-Eva P., Sanders M. D., Govan G. G., Sweeney M. G., Da Costa J., Harding A. E. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995 Apr;118(Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- Rizzo J. F., 3rd Adenosine triphosphate deficiency: a genre of optic neuropathy. Neurology. 1995 Jan;45(1):11–16. doi: 10.1212/wnl.45.1.11. [DOI] [PubMed] [Google Scholar]
- Smith K. J. Conduction properties of central demyelinated and remyelinated axons, and their relation to symptom production in demyelinating disorders. Eye (Lond) 1994;8(Pt 2):224–237. doi: 10.1038/eye.1994.51. [DOI] [PubMed] [Google Scholar]
- Taylor R. W., Birch-Machin M. A., Bartlett K., Lowerson S. A., Turnbull D. M. The control of mitochondrial oxidations by complex III in rat muscle and liver mitochondria. Implications for our understanding of mitochondrial cytopathies in man. J Biol Chem. 1994 Feb 4;269(5):3523–3528. [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Molecular dissection of the myelinated axon. Ann Neurol. 1993 Feb;33(2):121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]