Abstract
AIM—To describe the early formation of drusen and their relation to normal aging changes at the macula and to the development of age related maculopathy (ARM). METHOD—Histopathological features of 353 eyes without histological evidence of ARM are described and correlated with the clinical appearance. In addition, 45 of these eyes were examined by transmission electron microscopy. RESULTS—Drusen were detected histopathologically in 177 (50%) eyes but were seen clinically in only 34% of these. Drusen were mainly small hard drusen with an occasional soft distinct drusen: no soft indistinct drusen were seen. Only those drusen deposits larger than 25-30 µm in diameter were detectable clinically. Preclinical drusen in eyes with only an occasional drusen were seen on electron microscopy as entrapment sites of coated membrane bound bodies which formed adjacent to the inner collagenous zone of Bruch's membrane. In contrast, preclinical drusen deposits in eyes with many drusen were seen as accumulations of amorphous material which appeared hyalinised by light microscopy. A distinct feature were rows of dense hyalinised microdrusen (1-2 µm in diameter), over which larger globular hyalinised drusen formed. CONCLUSION—Histological and ultrastructural examination can recognise and distinguish the earliest drusen formed as a result of normal aging from those associated with ARM. In eyes without diffuse deposits, histologically all drusen were of the hard hyalinised variety or their derivatives; no soft drusen composed of membranous debris were found. These findings support and explain those of other authors who do not consider the presence of a few small hard drusen to be a risk factor for the development of ARM. Keywords: aging; macular degeneration; retinal drusen; entrapment
Full Text
The Full Text of this article is available as a PDF (502.2 KB).
Figure 1 .
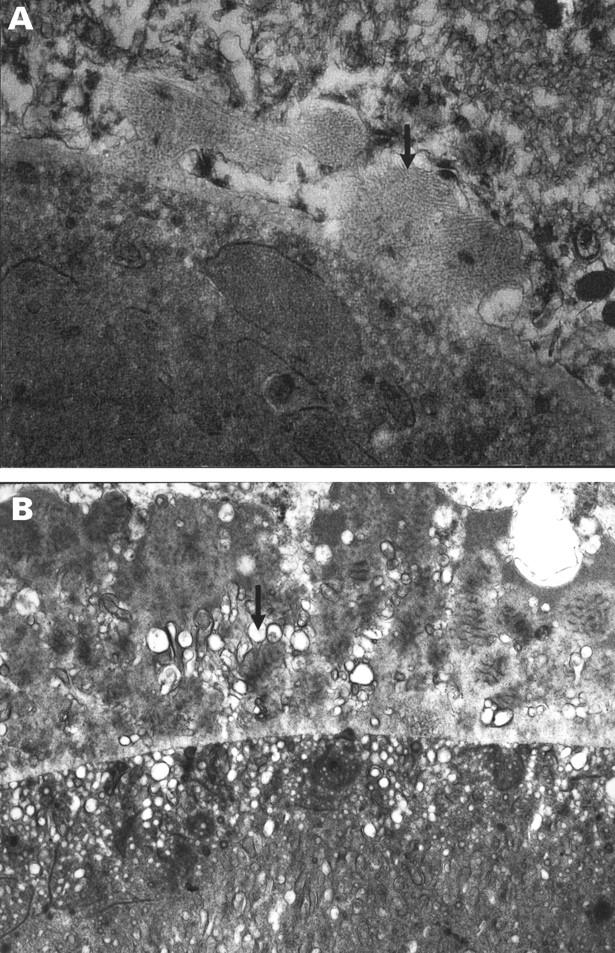
Basal lamina deposit over drusen of mixed composition. (A) Earliest BLD from a group 2 eye consisting of irregular nodules of fibrillar material (arrow) continuous with the RPE basement membrane; there is no membranous debris. (B) In comparison, later BLD seen here in an eye from group 4 consists of fibrillar, amorphous, and long spacing collagen material with a palisading or banded appearance. Membranous debris (arrow) is interspersed between the strands of BLD and within the drusen. (A) A 56 year old man with normal fundal appearance, vision 6/6. (B) A 68 year old man with soft indistinct (membranous) drusen and a few small hard drusen, vision 6/6. (A) ×21 600 (B) ×11 400.
Figure 2 .
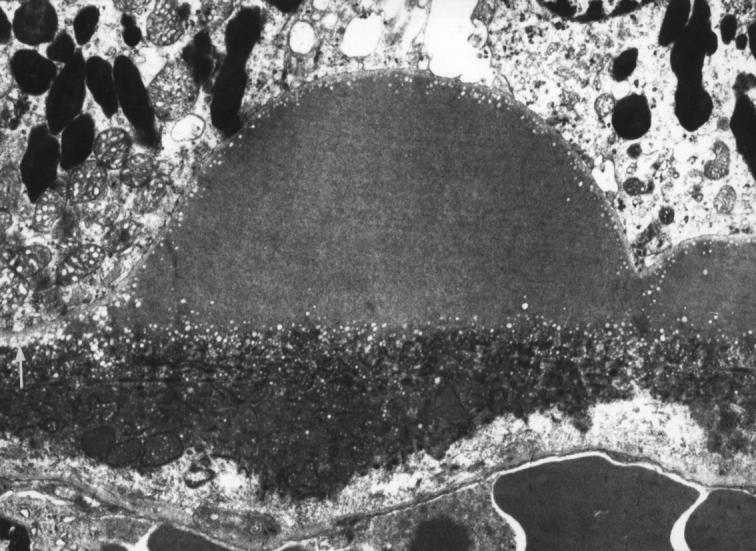
Electron lucent droplets forming a layer three to four rows deep beneath the RPE basement membrane (white arrow) and also beneath and over the apex of a small clinical drusen composed of amorphous material. 79 year old man with normal fundal appearance ×6430.
Figure 3 .
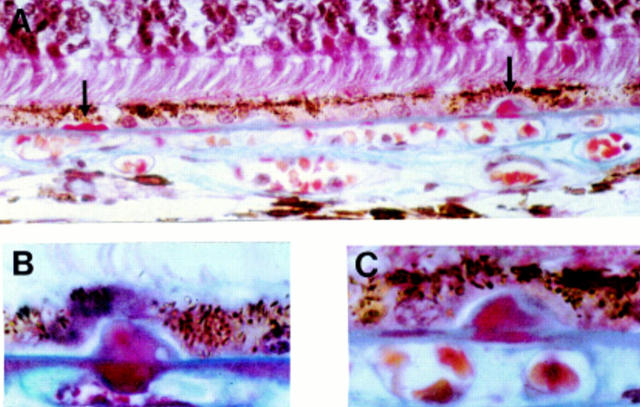
Preclinical hyalinised drusen formation in eyes with normal fundal appearance. (A) Left arrow shows row of three drusen, all less than half the height of an RPE cell. Right arrow shows single drusen one RPE cell high; (B) note the "root-like" extension of the hyalinisation into the outer collagenous zone; (C) higher magnification of right drusen shown in (A). Note the regions of blue and red staining within the drusen. (A) and (C) a 42 year old man, vision 6/6, (B) a 75 year old man, vision 6/6. Picro-Mallory stain (A) ×300, (B) and (C) ×500.
Figure 4 .
Preclinical small globular drusen and microdrusen. Microdrusen each around 1-2 µm in size forming a row (between arrows) of around 125-150 µm. Open arrow marks a cluster of two small preclinical drusen. A 74 year old man with significant numbers of small hard drusen and drusen clusters, vision 6/6. A 0.5 µm plastic section methylene blue, ×420.
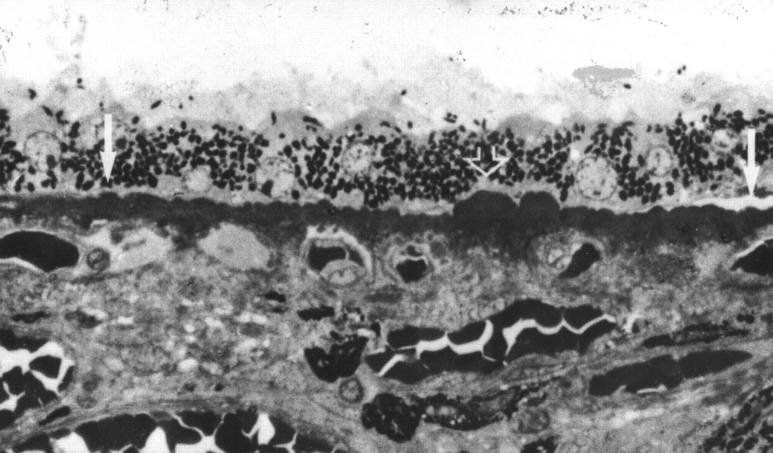
Figure 5 .
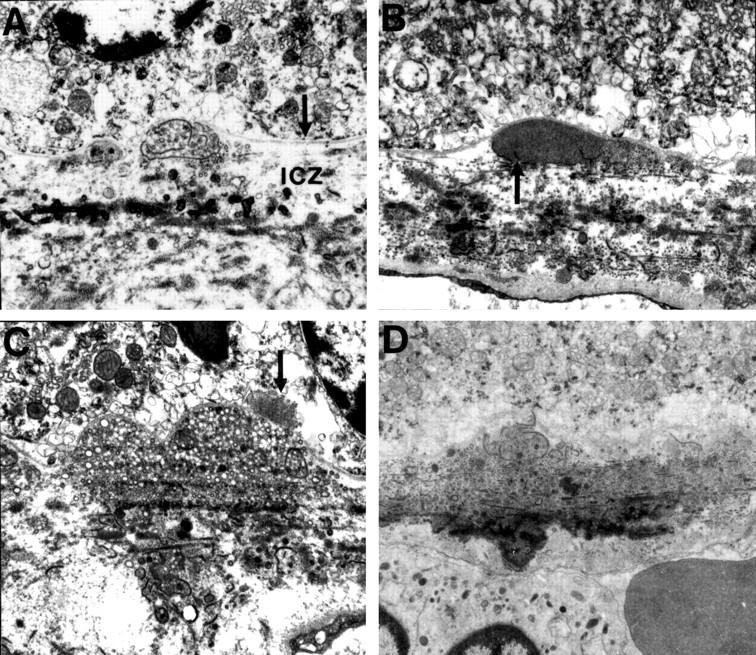
Preclinical drusen composed of entrapment sites, (A) and (B) only detectable on EM. (A) Intact (left) and ruptured (right) coated membrane bound bodies (CMBB) situated between the inner collagenous zone (icz) and basement membrane of the RPE (arrow). The ruptured CMBB have released their contents into the underlying collagenous zones. (B) Amorphous material that appears to be derived from the rupture or breakdown of CMBB. Arrow points to fragments of CMBB. (C) Entrapment site composed predominantly of vesicular material which is also present in the collagenous zones. Arrow points to a patch of BLD over the drusen. (D) Entrapment site composed of ruptured CMBB. Note the electron dense amorphous material in the outer collagenous zone giving the appearance of a root-like extension beneath the drusen similar to Figure 1 (B). (A), (B), (C) A 60 year old man with normal fundus appearance, vision 6/6. (D) A 43 year old man with normal fundal appearance, vision 6/6. (A) ×8880, (B) ×9700, (C) ×8000, (D) ×6250.
Figure 6 .
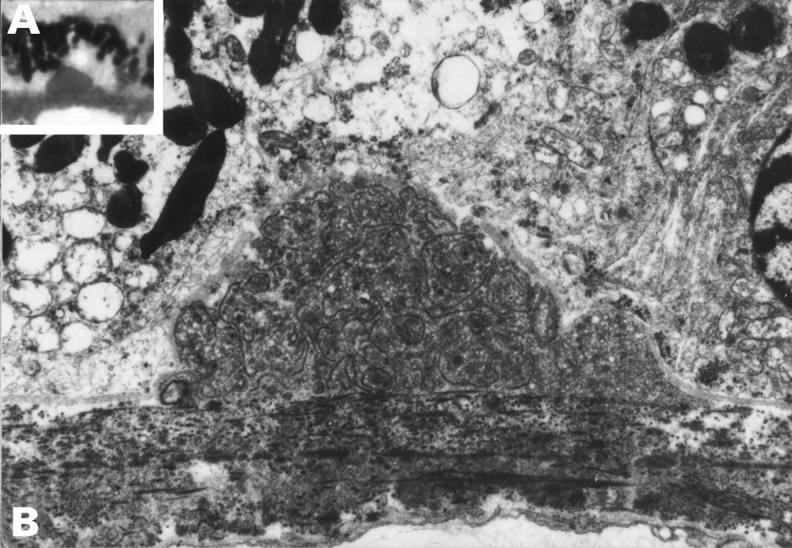
Preclinical drusen, entrapment site. This drusen appeared hyalinised on light microscopy (inset A) but EM (B) shows that it is an entrapment site composed of coated membrane bound bodies. A 50 year old man with normal fundal appearance, vision 6/6. (A) ×500, (B) ×5600.
Figure 7 .
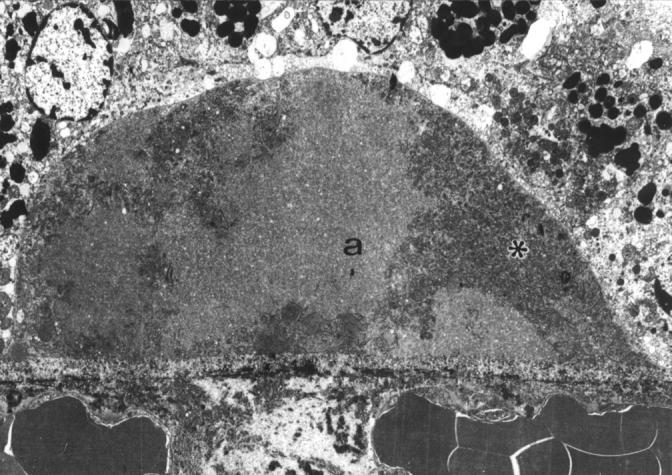
Preclinical drusen of mixed composition. EM shows an entrapment site of coated membrane bound bodies (asterisk) which has enlarged by the addition of amorphous material (a). This drusen would stain heterogeneously on LM with picro-Mallory. A 58 year old man with scattered small hard drusen, vision 6/6. ×3500
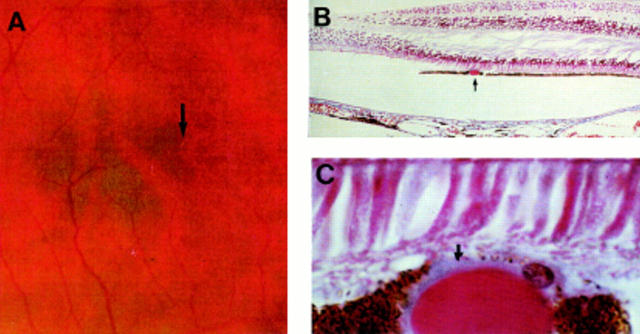
Figure 8 .
Smallest drusen detectable clinically. (A) Colour fundus photograph showing small hard drusen near the fovea (arrow). (B) LM section through fovea showing hyalinised drusen (arrow); (C) high power LM. The drusen measures 30 µm in diameter. Note the blue staining BLD (arrow) over the surface of the drusen. The overlying RPE cells are displaced and attenuated. Right eye of 71 year old man, vision 6/6. (B) and (C) picro-Mallory, (B) ×75. (C) ×500.
Figure 9 .
Clinical drusen measuring 60 µm in diameter. Note that the hyalinised drusen has formed over a row of dense microdrusen (arrow) which continues beyond the drusen margin to the right. Compare with the normal Bruch's membrane on the left (open arrowhead). There is a small amount of BLD (asterisk) over the apex of the drusen. A 74 year old man with significant numbers of small hard drusen and drusen clusters, vision 6/6. EM ×1785.
Figure 10 .
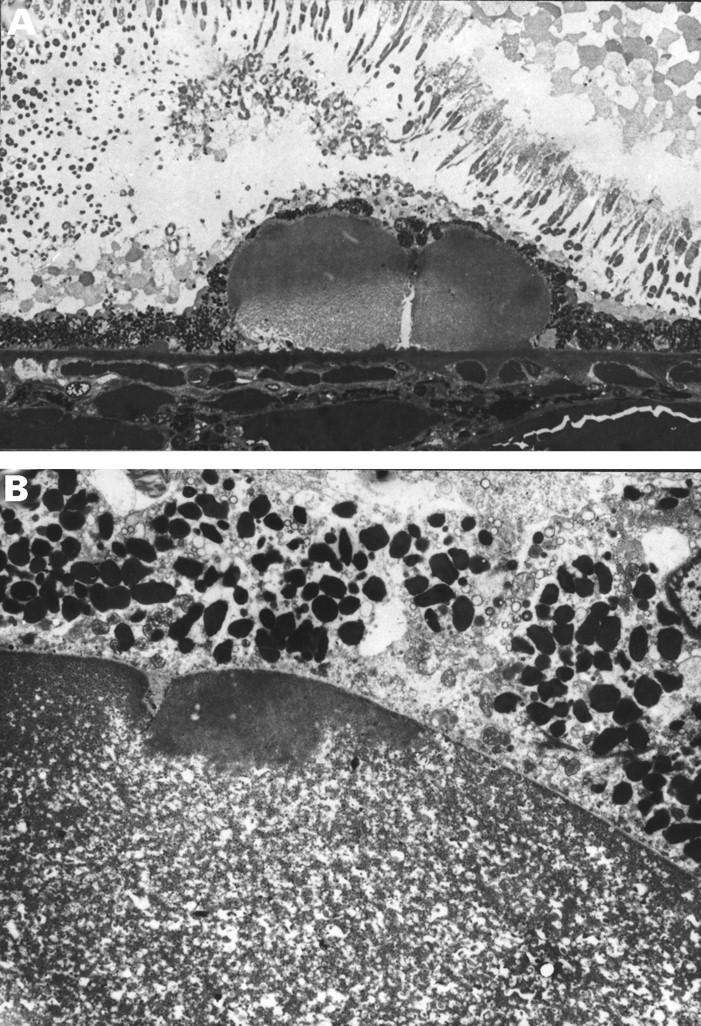
Dispersal of hyalinised clinical drusen. (A) The contents of this cluster of two drusen appear paler, beginning from the base. Note the row of dense microdrusen lying beneath the drusen. (B) On EM there is dispersal of the amorphous contents giving a flocculant appearance. A rim of amorphous material remains over the apex. A 74 year old man with significant numbers of small hard drusen and drusen clusters, vision 6/6. (A) 0.5 µm plastic section methylene blue, ×300, (B) ×3690.
Figure 11 .
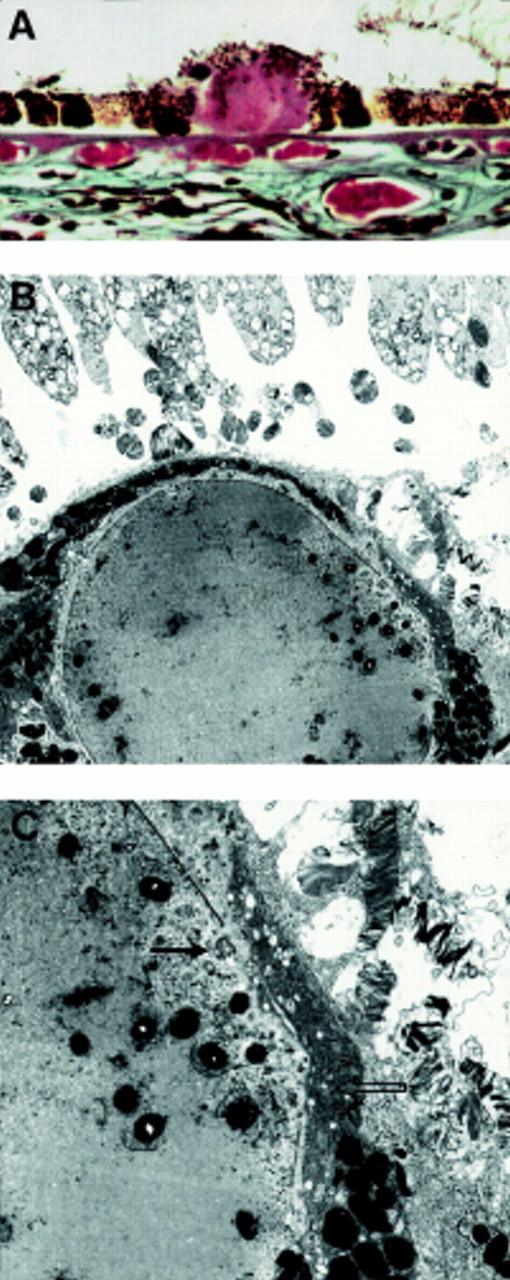
Degenerative changes in a clinical drusen. (A) LM shows outpouching from the smooth globular surface and pigment granules within the drusen; (B) low; and (C) high magnification EM showing dark and condensed RPE cells overlying the drusen (open arrow). A fragment of degenerate RPE cytoplasm has herniated through a break in the RPE basement membrane into the drusen (arrow). A 73 year old man with less than five small hard drusen, vision 6/6. (A) Picro-Mallory ×300, (B) ×2440, (C) ×10 030.
Figure 12 .
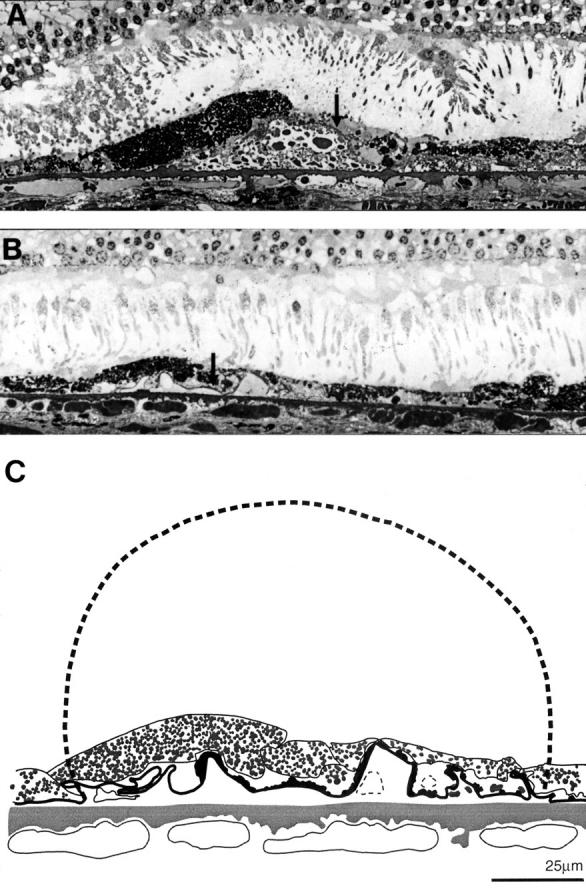
Drusen regression and disappearance. (A) The drusen contents have become coarsely granular. The overlying RPE is degenerate; note the large hyperpigmented cell (asterisk) next to an area of RPE attenuation and cell loss (arrow). (B) The RPE basement membrane appears collapsed and has become infolded (arrow) at the site where a granular drusen has disappeared; (C) overlay taken from EM of section shown in (B). The collapsed basement membrane has been measured. The broken line shows the drusen, 125 µm in diameter, which would correspond to this length. Drusen of this size were seen in other sections from this eye. A 74 year old man with significant numbers of small hard drusen and drusen clusters, vision 6/6. 0.5 µm plastic section methylene blue, ×370.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. C. Doyne Lecture. Pathogenesis of retinal pigment epithelial detachment in the elderly; the relevance of Bruch's membrane change. Eye (Lond) 1991;5(Pt 1):1–12. doi: 10.1038/eye.1991.2. [DOI] [PubMed] [Google Scholar]
- Bressler N. M., Bressler S. B., West S. K., Fine S. L., Taylor H. R. The grading and prevalence of macular degeneration in Chesapeake Bay watermen. Arch Ophthalmol. 1989 Jun;107(6):847–852. doi: 10.1001/archopht.1989.01070010869032. [DOI] [PubMed] [Google Scholar]
- Bressler N. M., Munoz B., Maguire M. G., Vitale S. E., Schein O. D., Taylor H. R., West S. K. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Waterman study. Arch Ophthalmol. 1995 Mar;113(3):301–308. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- Burns R. P., Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch's membrane. Trans Am Ophthalmol Soc. 1980;78:206–225. [PMC free article] [PubMed] [Google Scholar]
- Coffey A. J., Brownstein S. The prevalence of macular drusen in postmortem eyes. Am J Ophthalmol. 1986 Aug 15;102(2):164–171. doi: 10.1016/0002-9394(86)90138-8. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L., Burns R. P., Gao C. L. Age-related macular changes in humans over 90 years old. Am J Ophthalmol. 1990 Mar 15;109(3):265–278. doi: 10.1016/s0002-9394(14)74549-0. [DOI] [PubMed] [Google Scholar]
- Gass J. D., Jallow S., Davis B. Adult vitelliform macular detachment occurring in patients with basal laminar drusen. Am J Ophthalmol. 1985 Apr 15;99(4):445–459. doi: 10.1016/0002-9394(85)90012-1. [DOI] [PubMed] [Google Scholar]
- Green W. R., Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993 Oct;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Hogan M. J. Bruch's membrane and disease of the macula. Role of elastic tissue and collagen. Trans Ophthalmol Soc U K. 1967;87:113–161. [PubMed] [Google Scholar]
- Ishibashi T., Patterson R., Ohnishi Y., Inomata H., Ryan S. J. Formation of drusen in the human eye. Am J Ophthalmol. 1986 Mar 15;101(3):342–353. doi: 10.1016/0002-9394(86)90830-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Sorgente N., Patterson R., Ryan S. J. Pathogenesis of drusen in the primate. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):184–193. [PubMed] [Google Scholar]
- Killingsworth M. C. Age-related components of Bruch's membrane in the human eye. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Jensen S. C., Meuer S. M. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997 Jan;104(1):7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Linton K. L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992 Jun;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- Löffler K. U., Lee W. R. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224(6):493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Smith W., Attebo K., Wang J. J. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995 Oct;102(10):1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- Mullins R. F., Johnson L. V., Anderson D. H., Hageman G. S. Characterization of drusen-associated glycoconjugates. Ophthalmology. 1997 Feb;104(2):288–294. doi: 10.1016/s0161-6420(97)30322-4. [DOI] [PubMed] [Google Scholar]
- Sarks J. P., Sarks S. H., Killingsworth M. C. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Sarks J. P., Sarks S. H., Killingsworth M. C. Evolution of soft drusen in age-related macular degeneration. Eye (Lond) 1994;8(Pt 3):269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- Sarks S. H. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976 May;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks S. H. Council Lecture. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980 May;8(2):117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Spraul C. W., Grossniklaus H. E. Characteristics of Drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997 Feb;115(2):267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- Starita C., Hussain A. A., Patmore A., Marshall J. Localization of the site of major resistance to fluid transport in Bruch's membrane. Invest Ophthalmol Vis Sci. 1997 Mar;38(3):762–767. [PubMed] [Google Scholar]
- Vingerling J. R., Dielemans I., Hofman A., Grobbee D. E., Hijmering M., Kramer C. F., de Jong P. T. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995 Feb;102(2):205–210. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- el Baba F., Green W. R., Fleischmann J., Finkelstein D., de la Cruz Z. C. Clinicopathologic correlation of lipidization and detachment of the retinal pigment epithelium. Am J Ophthalmol. 1986 May 15;101(5):576–583. doi: 10.1016/0002-9394(86)90948-7. [DOI] [PubMed] [Google Scholar]