Abstract
AIMS—Examination of the expression of the mutated allele of myotonic dystrophy protein kinase gene and lens epithelial cell changes in patients with myotonic dystrophy. METHODS—Six eyes from three patients with myotonic dystrophy underwent cataract surgery. The lens epithelium was photographed to examine the morphological changes. mRNAs were extracted to determine myotonic dystrophy protein kinase gene expression in the lens epithelium and peripheral blood. Age matched lens epithelial cells from senile cataracts were used as controls. RESULTS—All eyes showed iridescent or posterior subcapsular lens opacity. The expression of the myotonic dystrophy protein kinase gene with trinucleotide repeat expansion was evaluated by reverse transcriptase polymerase chain reaction, Southern blotting, and sequence analysis. Lens epithelial cell densities were extremely reduced in the patients compared with the control group. CONCLUSION—To the authors' knowledge, this is the first report to describe the relation between lens epithelial cell changes and mutated gene expression in patients with myotonic dystrophy. The gene may be mitotically unstable in the lens epithelial cells; it may influence cell density and lens epithelial function, and it may lead to the development of typical subcapsular lens opacity. Keywords: lens epithelial cell; myotonic dystrophy; RT-PCR
Full Text
The Full Text of this article is available as a PDF (215.0 KB).
Figure 1 .
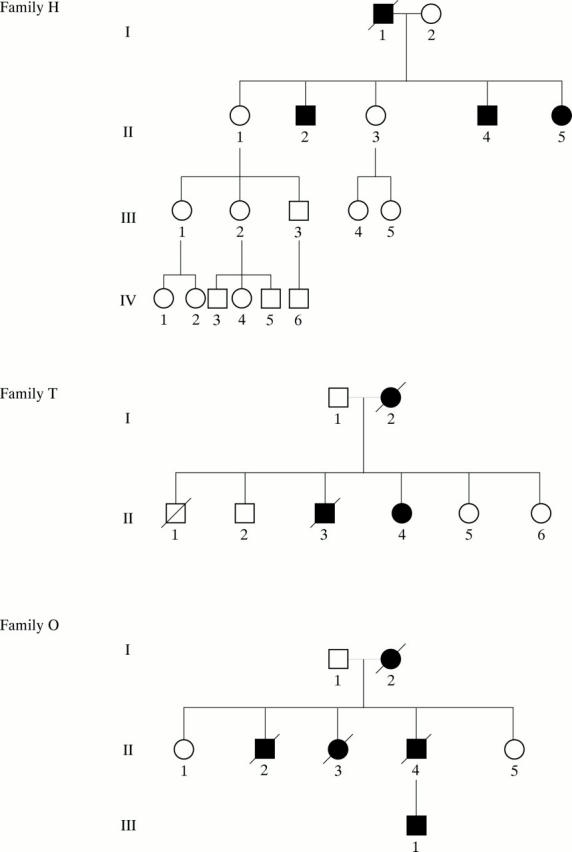
Pedigrees of three families showing generations (roman numerals) of affected (closed symbols) and unaffected (open symbols) members. Squares indicate male members; circles, female; and slash, deceased.
Figure 2 .
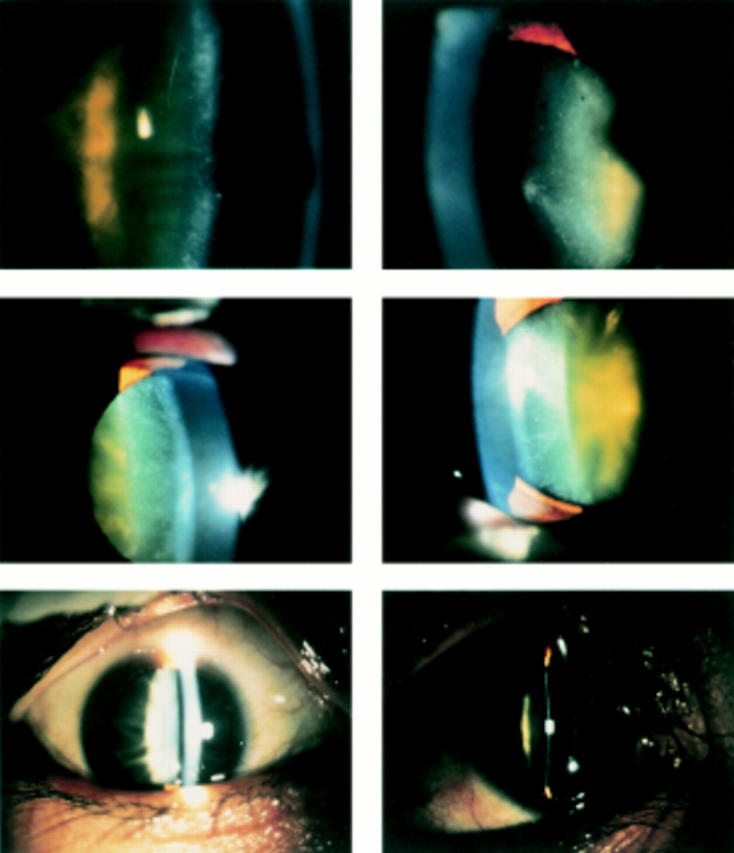
Photographs of cataracts were taken in affected patients. Patient H-II-2 (top, right and left are right eye and left eye, respectively) is a 59 year old man; patient T-II-4 (middle; right and left eyes) is a 58 year old woman; and patient O-III-1 (bottom; right and left eyes) is a 33 year old man. All showed either cortical iridescent opacity or posterior cortical opacity. Patients are described in the text.
Figure 3 .
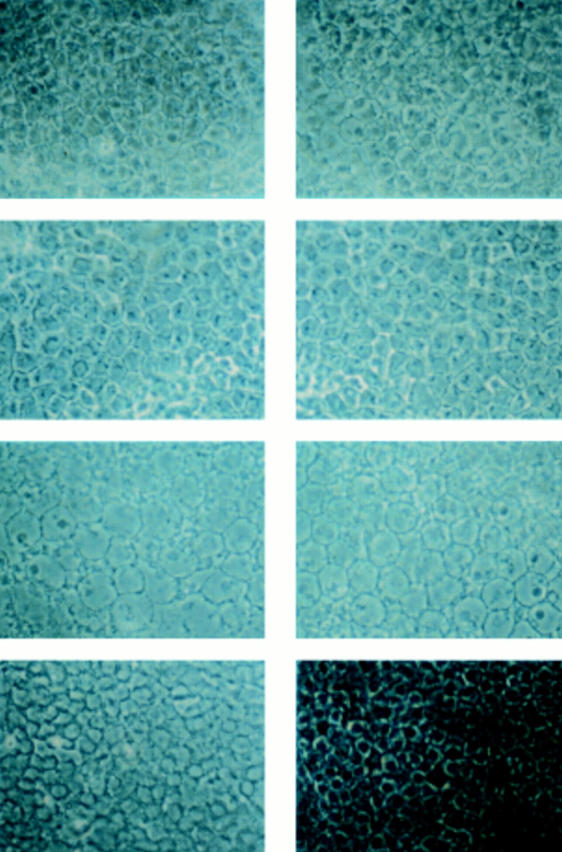
Examination of lens epithelial cell density reveals that all MD patients with cataract show extremely decreased cell densities, compared with the age matched normal subject. Lens epithelium of patient T-II-4 (top; right and left are right eye and left eye, respectively), a 58 year old woman; patient H-II-2 (second from top; right and left eyes), a 59 year old man; patient O-III-1 (second from bottom; right and left eyes), a 33 year old man; control subject with senile cataract (bottom right), a 78 year old man and also other control subject with senile cataract (bottom left), a 58 year old man. Actual cell density is shown in Table 1.
Figure 4 .
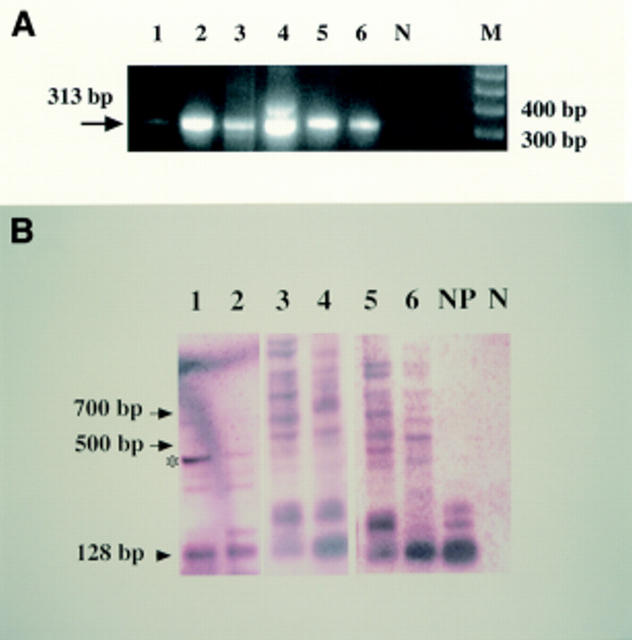
Results of reverse transcriptase polymerase chain reaction (RT-PCR) and Southern blotting analysis from affected patients in the families. (A) Results of β actin from lens epithelial cells and peripheral blood from patients. Patient H-II-2 (Nos 1 and 2 indicate lens epithelial cells and peripheral blood, respectively) is a 59 year old man; patient T-II-4 (Nos 3 and 4, same as above) is a 58 year old woman; and patient O-III-1 (Nos 5 and 6, same as above) is a 33 year old man. The negative control is indicated by N. M is a marker of the 100 base pair ladder. (B) Results of myotonic dystrophy protein kinase gene. Normal band is indicated by arrowhead; extended bands, arrows. The normal control (NP) show no mutated band; conversely, mutated bands occur in all affected members examined. The extended band, which was shown by an asterisk, was subcloned and sequenced.
Figure 5 .
Result of the sequence of the subcloned DNA, which was amplified from the cDNA of the lens epithelium in patient T-II-4 (shown by an asterisk in Fig 4B[f4}. Abnormal CAG repeat, the reverse sequence of CTG, was observed in the amplified DNA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Abe K., Aoki M., Itoyama Y., Tamai M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch Ophthalmol. 1997 Feb;115(2):231–236. doi: 10.1001/archopht.1997.01100150233013. [DOI] [PubMed] [Google Scholar]
- Abe T., Durlu Y. K., Tamai M. The properties of retinal pigment epithelial cells in proliferative vitreoretinopathy compared with cultured retinal pigment epithelial cells. Exp Eye Res. 1996 Aug;63(2):201–210. doi: 10.1006/exer.1996.0109. [DOI] [PubMed] [Google Scholar]
- Abe T., Tsuchida K., Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996 Jul;234(7):419–424. doi: 10.1007/BF02539407. [DOI] [PubMed] [Google Scholar]
- Bourne W. M., Kaufman H. E. Specular microscopy of human corneal endothelium in vivo. Am J Ophthalmol. 1976 Mar;81(3):319–323. doi: 10.1016/0002-9394(76)90247-6. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Burian H. M., Burns C. A. Ocular changes in myotonic dystrophy. Am J Ophthalmol. 1967 Jan;63(1):22–34. doi: 10.1016/0002-9394(67)90577-6. [DOI] [PubMed] [Google Scholar]
- Carango P., Noble J. E., Marks H. G., Funanage V. L. Absence of myotonic dystrophy protein kinase (DMPK) mRNA as a result of a triplet repeat expansion in myotonic dystrophy. Genomics. 1993 Nov;18(2):340–348. doi: 10.1006/geno.1993.1474. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara T. Panoramic specular microscopy of lens epithelial cells in the postoperative pseudophakic eye. J Cataract Refract Surg. 1992 Nov;18(6):589–593. doi: 10.1016/s0886-3350(13)80449-x. [DOI] [PubMed] [Google Scholar]
- Harper P. S., Dyken P. R. Early-onset dystrophia myotonica. Evidence supporting a maternal environmental factor. Lancet. 1972 Jul 8;2(7767):53–55. doi: 10.1016/s0140-6736(72)91548-6. [DOI] [PubMed] [Google Scholar]
- Jansen G., Groenen P. J., Bächner D., Jap P. H., Coerwinkel M., Oerlemans F., van den Broek W., Gohlsch B., Pette D., Plomp J. J. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996 Jul;13(3):316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Kidd A., Turnpenny P., Kelly K., Clark C., Church W., Hutchinson C., Dean J. C., Haites N. E. Ascertainment of myotonic dystrophy through cataract by selective screening. J Med Genet. 1995 Jul;32(7):519–523. doi: 10.1136/jmg.32.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimizuka Y., Kiyosawa M., Tamai M., Takase S. Retinal changes in myotonic dystrophy. Clinical and follow-up evaluation. Retina. 1993;13(2):129–135. [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- Konofsky K., Naumann G. O., Guggenmoos-Holzmann I. Cell density and sex chromatin in lens epithelium of human cataracts. Quantitative studies in flat preparation. Ophthalmology. 1987 Jul;94(7):875–880. doi: 10.1016/s0161-6420(87)33543-2. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Martorell L., Martinez J. M., Carey N., Johnson K., Baiget M. Comparison of CTG repeat length expansion and clinical progression of myotonic dystrophy over a five year period. J Med Genet. 1995 Aug;32(8):593–596. doi: 10.1136/jmg.32.8.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiner A., Wolf C., Carey N., Okitsu A., Johnson K., Shelbourne P., Kunath B., Sauermann W., Thiele H., Kupferling P. Direct molecular analysis of myotonic dystrophy in the German population: important considerations in genetic counselling. J Med Genet. 1995 Aug;32(8):645–649. doi: 10.1136/jmg.32.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Aquavella J. V., Goldberg S. H., Berk S. L. Pseudophakic bullous keratopathy. Relationship to preoperative corneal endothelial status. Ophthalmology. 1984 Oct;91(10):1135–1140. [PubMed] [Google Scholar]
- Reddy S., Smith D. B., Rich M. M., Leferovich J. M., Reilly P., Davis B. M., Tran K., Rayburn H., Bronson R., Cros D. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat Genet. 1996 Jul;13(3):325–335. doi: 10.1038/ng0796-325. [DOI] [PubMed] [Google Scholar]
- Saitoh J., Nishi O., Hitani H. [Cell density and hexagonality of lens epithelium in human cataracts]. Nippon Ganka Gakkai Zasshi. 1990 Feb;94(2):176–180. [PubMed] [Google Scholar]
- Standard for clinical electroretinography. International Standardization Committee. Arch Ophthalmol. 1989 Jun;107(6):816–819. doi: 10.1001/archopht.1989.01070010838024. [DOI] [PubMed] [Google Scholar]
- Streeten B. W., Eshaghian J. Human posterior subcapsular cataract. A gross and flat preparation study. Arch Ophthalmol. 1978 Sep;96(9):1653–1658. doi: 10.1001/archopht.1978.03910060279020. [DOI] [PubMed] [Google Scholar]
- Tohgi H., Kawamorita A., Utsugisawa K., Yamagata M., Sano M. Muscle histopathology in myotonic dystrophy in relation to age and muscular weakness. Muscle Nerve. 1994 Sep;17(9):1037–1043. doi: 10.1002/mus.880170911. [DOI] [PubMed] [Google Scholar]
- VON SALLMANN L. The lens epithelium in the pathogenesis of cataract; the XIII Edward Jackson Memorial lecture. Am J Ophthalmol. 1957 Aug;44(2):159–170. doi: 10.1016/0002-9394(57)90001-6. [DOI] [PubMed] [Google Scholar]
- Vasavada A. R., Cherian M., Yadav S., Rawal U. M. Lens epithelial cell density and histomorphological study in cataractous lenses. J Cataract Refract Surg. 1991 Nov;17(6):798–804. doi: 10.1016/s0886-3350(13)80415-4. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wong L. J., Ashizawa T., Monckton D. G., Caskey C. T., Richards C. S. Somatic heterogeneity of the CTG repeat in myotonic dystrophy is age and size dependent. Am J Hum Genet. 1995 Jan;56(1):114–122. [PMC free article] [PubMed] [Google Scholar]