Abstract
AIM—To determine the spatial and temporal changes in the staining pattern of the VEGF receptors FLT-1, KDR, and the putative receptor FLT-4 during the pathogenesis of diabetic retinopathy. METHODS—Immunohistochemical localisation of VEGF receptors, using antibodies against FLT-1, FLT-4, and KDR, was carried out on specimens of normal human retina (n=10), diabetic retinas (a) with no overt retinopathy (n=12), (b) with intraretinal vascular abnormalities but no proliferative retinopathy (n=5), (c) with active proliferative retinopathy (n=6), and (d) with no residual proliferative retinopathy after scatter photocoagulation therapy (n=14), and surgically excised diabetic fibrovascular membranes (n=11). The degree and pattern of immunostaining was recorded. RESULTS—FLT-1 staining was apparent in the retinas from both non-diabetic and diabetic retinas; weak to moderate staining was generally confined to the inner nuclear layer, the ganglion cell layer, and the retinal vessels during all stages of the disease process. Staining of the retinal vessels was raised in diabetic tissue compared with non-diabetic tissue. The preretinal vessels of the diabetic subjects stained moderately to intensely for FLT-1. In contrast with FLT-1 staining minimal immunostaining for KDR was demonstrated in the non-diabetic eyes and the unlasered eyes; however, weak staining for KDR was observed in the inner nuclear layer and the ganglion cell layer of the unlasered eyes with diabetic changes. In those retinas with preretinal neovascularisation KDR immunoreactivity was moderate to intense in the intra- and preretinal vessels. However, in the excised membranes, where the vessels may have been in a quiescent state, the levels of KDR were weak to moderate. After apparently successful laser treatment KDR staining was reduced in the intraretinal vessels. Minimal FLT-4 staining was observed throughout normal eyes while weak to moderate FLT-4 staining was generally confined to the inner nuclear layer and the ganglion cell layer of the unlasered diabetic eyes. Weak to moderate levels of FLT-4 staining were observed in the intraretinal vessels except after apparently successful laser treatment where reduced levels of staining were observed. Weak to moderate staining was observed in the preretinal vessels. CONCLUSIONS—This study supports a role for FLT-1, KDR, and possibly FLT-4 in the pathogenesis of diabetic retinopathy; however, their specific roles in the progression of the disease may differ. Keywords: vascular endothelial growth factor; VEGF receptors; diabetic retinopathy
Full Text
The Full Text of this article is available as a PDF (218.8 KB).
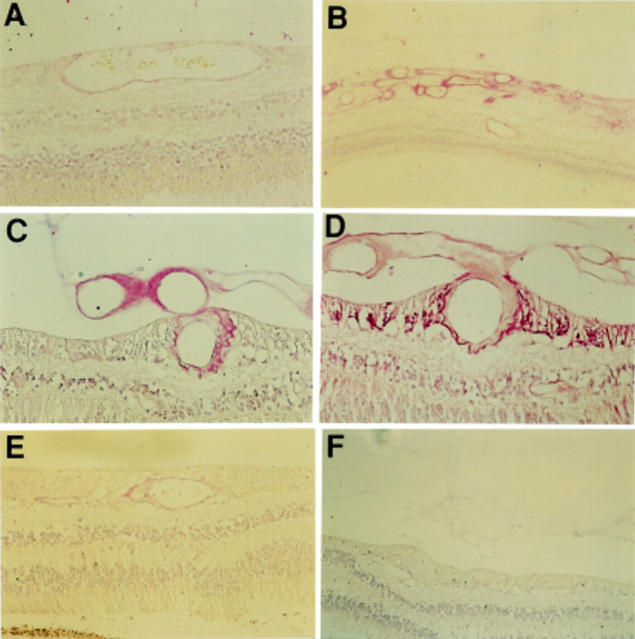
Figure 1 .
Photomicrographs demonstrating FLT-1 immunostaining of non-diabetic retina (A), diabetic retina with no obvious retinopathy (B), diabetic retina with obvious intraretinal vascular changes but no evidence of PDR (C), the same retina stained with GFAP (D), diabetic retina with PDR (E, F, G), diabetic retina post laser but with no residual PDR (H). Immunostaining for FLT-1 was greatest in the diabetic tissue compared with non-diabetic tissue. Increased staining was generally confined to the inner nuclear layer and the ganglion cell layer. Magnification A, G ×94; B, C, E ×118; D ×156; F ×378; H ×236.
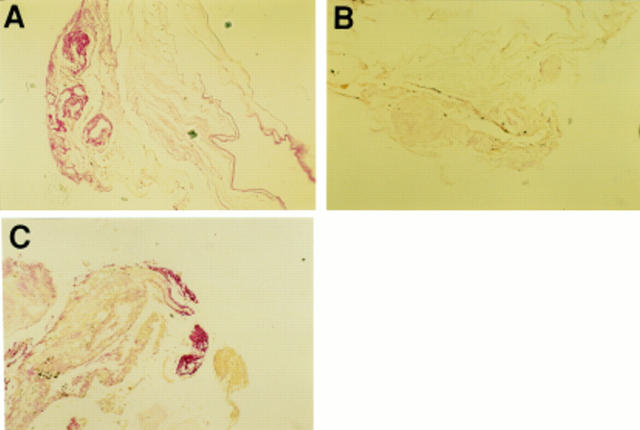
Figure 2 .
Photomicrographs demonstrating FLT-1 immunostaining of an excised fibrovascular membrane (A). Immunoreactivity for FLT-1 was abolished in a control specimen of excised membrane processed with omission of the primary antibody (B) and GFAP staining of the same excised membrane (C). Intense staining was observed around the vessels in excised membranes. Magnification A, B, C ×60.
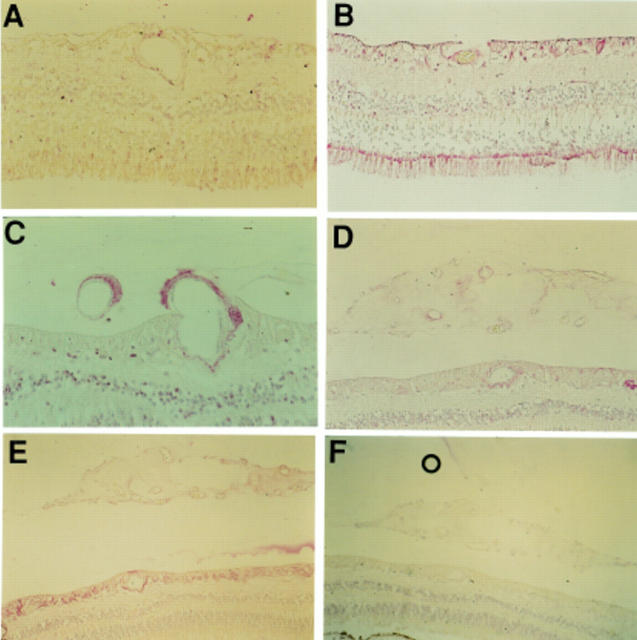
Figure 3 .
Photomicrographs demonstrating KDR immunostaining of non-diabetic retina (A), diabetic retina with PDR (B, C), the same retina stained with GFAP (D), and diabetic retina post laser but with no residual PDR (E). Immunostaining for KDR was greatest in the diabetic tissue with PDR and was minimal in most other diabetic tissue. Interestingly, immunostaining in the diabetic retinas which had undergone apparently successful laser treatment was reduced compared with the staining intensity in the retinas with PDR. Immunoreactivity for KDR was abolished in a control specimen of PDR retina processed with omission of the primary antibody (F). Magnification = A, C, D ×156; B ×60; E ×118; F ×78.
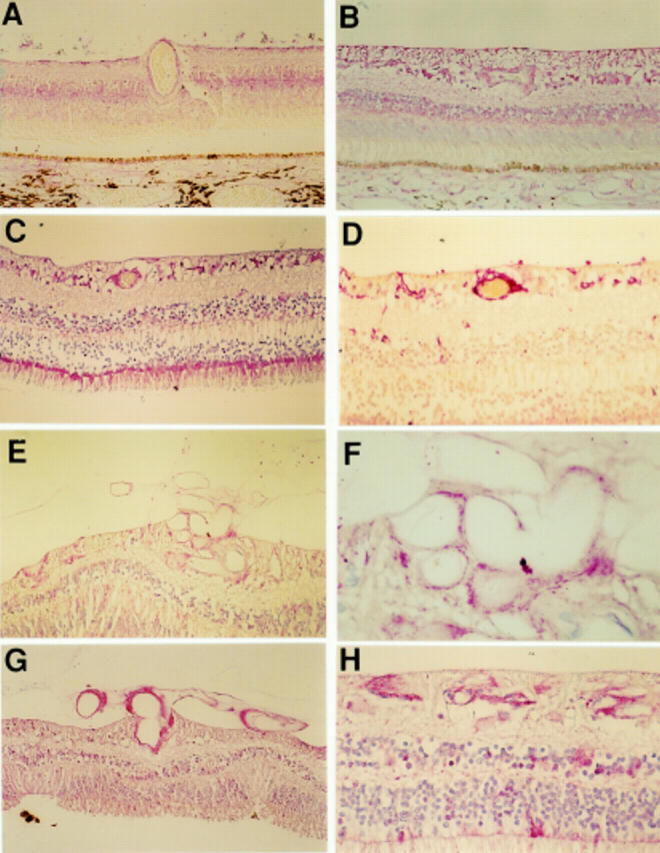
Figure 4 .
Photomicrographs demonstrating FLT-4 staining of normal retina (A), diabetic retina with obvious vascular intraretinal changes but no evidence of PDR (B), diabetic retina with PDR (C, D) and the same retina stained with GFAP (E). Immunostaining for FLT-4 was raised in diabetic tissue compared with non-diabetic tissue. Immunostaining was intermediate in the PDR specimens. Immunoreactivity for FLT-4 was abolished in a control specimen of PDR retina processed with omission of the primary antibody (F). Magnification A, D ×156; B ×118; C ×94; E, F ×60.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis A. P., Shima D. T., Tolentino M. J., Gragoudas E. S., Ferrara N., Folkman J., D'Amore P. A., Miller J. W. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996 Jan;114(1):66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Pierce E. A., Foley E. D., Takagi H., Chen H., Riddle L., Ferrara N., King G. L., Smith L. E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R. H., Frank R. N., Kennedy A., Eliott D., Puklin J. E., Abrams G. W. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):36–47. [PubMed] [Google Scholar]
- Boocock C. A., Charnock-Jones D. S., Sharkey A. M., McLaren J., Barker P. J., Wright K. A., Twentyman P. R., Smith S. K. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995 Apr 5;87(7):506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- Borg J. P., deLapeyrière O., Noguchi T., Rottapel R., Dubreuil P., Birnbaum D. Biochemical characterization of two isoforms of FLT4, a VEGF receptor-related tyrosine kinase. Oncogene. 1995 Mar 2;10(5):973–984. [PubMed] [Google Scholar]
- Boulton M. E., McLeod D., Garner A. Vasoproliferative retinopathies: clinical, morphogenetic and modulatory aspects. Eye (Lond) 1988;2 (Suppl):S124–S139. doi: 10.1038/eye.1988.139. [DOI] [PubMed] [Google Scholar]
- Boulton M., Foreman D., Williams G., McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998 May;82(5):561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G., Clauss M., Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995 Nov;204(3):228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Brogi E., Schatteman G., Wu T., Kim E. A., Varticovski L., Keyt B., Isner J. M. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996 Jan 15;97(2):469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Olbricht S. M., Berse B., Jackman R. W., Matsueda G., Tognazzi K. A., Manseau E. J., Dvorak H. F., Van de Water L. Overexpression of vascular permeability factor (VPF/VEGF) and its endothelial cell receptors in delayed hypersensitivity skin reactions. J Immunol. 1995 Mar 15;154(6):2801–2807. [PubMed] [Google Scholar]
- Chen Y. S., Hackett S. F., Schoenfeld C. L., Vinores M. A., Vinores S. A., Campochiaro P. A. Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br J Ophthalmol. 1997 Oct;81(10):919–926. doi: 10.1136/bjo.81.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G., Naik P., Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995 Dec;9(12):1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- Detmar M., Brown L. F., Claffey K. P., Yeo K. T., Kocher O., Jackman R. W., Berse B., Dvorak H. F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994 Sep 1;180(3):1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. The trigger for neovascularization in the eye. Lab Invest. 1995 Jun;72(6):615–618. [PubMed] [Google Scholar]
- Galland F., Karamysheva A., Pebusque M. J., Borg J. P., Rottapel R., Dubreuil P., Rosnet O., Birnbaum D. The FLT4 gene encodes a transmembrane tyrosine kinase related to the vascular endothelial growth factor receptor. Oncogene. 1993 May;8(5):1233–1240. [PubMed] [Google Scholar]
- Gitay-Goren H., Cohen T., Tessler S., Soker S., Gengrinovitch S., Rockwell P., Klagsbrun M., Levi B. Z., Neufeld G. Selective binding of VEGF121 to one of the three vascular endothelial growth factor receptors of vascular endothelial cells. J Biol Chem. 1996 Mar 8;271(10):5519–5523. doi: 10.1074/jbc.271.10.5519. [DOI] [PubMed] [Google Scholar]
- Gitay-Goren H., Soker S., Vlodavsky I., Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992 Mar 25;267(9):6093–6098. [PubMed] [Google Scholar]
- Guerrin M., Moukadiri H., Chollet P., Moro F., Dutt K., Malecaze F., Plouët J. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol. 1995 Aug;164(2):385–394. doi: 10.1002/jcp.1041640219. [DOI] [PubMed] [Google Scholar]
- Hewett P. W., Murray J. C. Coexpression of flt-1, flt-4 and KDR in freshly isolated and cultured human endothelial cells. Biochem Biophys Res Commun. 1996 Apr 25;221(3):697–702. doi: 10.1006/bbrc.1996.0659. [DOI] [PubMed] [Google Scholar]
- Jakeman L. B., Winer J., Bennett G. L., Altar C. A., Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992 Jan;89(1):244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Pajusola K., Kaipainen A., Chilov D., Lahtinen I., Kukk E., Saksela O., Kalkkinen N., Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996 Jan 15;15(2):290–298. [PMC free article] [PubMed] [Google Scholar]
- Kendall R. L., Wang G., Thomas K. A. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996 Sep 13;226(2):324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Khaliq A., Foreman D., Ahmed A., Weich H., Gregor Z., McLeod D., Boulton M. Increased expression of placenta growth factor in proliferative diabetic retinopathy. Lab Invest. 1998 Jan;78(1):109–116. [PubMed] [Google Scholar]
- Kolch W., Martiny-Baron G., Kieser A., Marmé D. Regulation of the expression of the VEGF/VPS and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995;36(2):139–155. doi: 10.1007/BF00666036. [DOI] [PubMed] [Google Scholar]
- Lutty G. A., McLeod D. S., Merges C., Diggs A., Plouét J. Localization of vascular endothelial growth factor in human retina and choroid. Arch Ophthalmol. 1996 Aug;114(8):971–977. doi: 10.1001/archopht.1996.01100140179011. [DOI] [PubMed] [Google Scholar]
- Malecaze F., Clamens S., Simorre-Pinatel V., Mathis A., Chollet P., Favard C., Bayard F., Plouet J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994 Nov;112(11):1476–1482. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Murata T., Ishibashi T., Khalil A., Hata Y., Yoshikawa H., Inomata H. Vascular endothelial growth factor plays a role in hyperpermeability of diabetic retinal vessels. Ophthalmic Res. 1995;27(1):48–52. doi: 10.1159/000267567. [DOI] [PubMed] [Google Scholar]
- Murata T., Nakagawa K., Khalil A., Ishibashi T., Inomata H., Sueishi K. The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest. 1996 Jan;74(1):68–77. [PubMed] [Google Scholar]
- Neufeld G., Tessler S., Gitay-Goren H., Cohen T., Levi B. Z. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res. 1994;5(1):89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yamagishi S., Harada S., Hayashi Y., Yamashima T., Yamashita J., Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995 Nov 24;270(47):28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- Oelrichs R. B., Reid H. H., Bernard O., Ziemiecki A., Wilks A. F. NYK/FLK-1: a putative receptor protein tyrosine kinase isolated from E10 embryonic neuroepithelium is expressed in endothelial cells of the developing embryo. Oncogene. 1993 Jan;8(1):11–18. [PubMed] [Google Scholar]
- Pe'er J., Folberg R., Itin A., Gnessin H., Hemo I., Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 1996 Mar;80(3):241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'er J., Shweiki D., Itin A., Hemo I., Gnessin H., Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995 Jun;72(6):638–645. [PubMed] [Google Scholar]
- Peters K. G., De Vries C., Williams L. T. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce E. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. P., Peters K. G., De Vries C., Ferrara N., Williams L. T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawano A., Takahashi T., Yamaguchi S., Aonuma M., Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996 Feb;7(2):213–221. [PubMed] [Google Scholar]
- Simorre-Pinatel V., Guerrin M., Chollet P., Penary M., Clamens S., Malecaze F., Plouet J. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3393–3400. [PubMed] [Google Scholar]
- Stone J., Itin A., Alon T., Pe'er J., Gnessin H., Chan-Ling T., Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995 Jul;15(7 Pt 1):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn L. M., McMahon G., App H., Schreck R., Kuchler W. R., Longhi M. P., Hui T. H., Tang C., Levitzki A., Gazit A. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996 Aug 1;56(15):3540–3545. [PubMed] [Google Scholar]
- Takagi H., King G. L., Aiello L. P. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996 Aug;45(8):1016–1023. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- Takagi H., King G. L., Ferrara N., Aiello L. P. Hypoxia regulates vascular endothelial growth factor receptor KDR/Flk gene expression through adenosine A2 receptors in retinal capillary endothelial cells. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1311–1321. [PubMed] [Google Scholar]
- Terman B., Khandke L., Dougher-Vermazan M., Maglione D., Lassam N. J., Gospodarowicz D., Persico M. G., Böhlen P., Eisinger M. VEGF receptor subtypes KDR and FLT1 show different sensitivities to heparin and placenta growth factor. Growth Factors. 1994;11(3):187–195. doi: 10.3109/08977199409046916. [DOI] [PubMed] [Google Scholar]
- Thieme H., Aiello L. P., Takagi H., Ferrara N., King G. L. Comparative analysis of vascular endothelial growth factor receptors on retinal and aortic vascular endothelial cells. Diabetes. 1995 Jan;44(1):98–103. doi: 10.2337/diab.44.1.98. [DOI] [PubMed] [Google Scholar]
- Thomas K. A. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996 Jan 12;271(2):603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Vaisman N., Gospodarowicz D., Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990 Nov 15;265(32):19461–19466. [PubMed] [Google Scholar]
- Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994 Oct 28;269(43):26988–26995. [PubMed] [Google Scholar]