Abstract
AIM—To evaluate the efficacy of autologous serum application for the treatment of dry eye in Sjögren's syndrome. METHODS—The stability of essential components (EGF, vitamin A, and TGF-β) in preserved serum were examined following preservation at 4°C and −20°C. In a primary clinical trial, 12 patients with Sjögren's syndrome were treated with autologous serum (diluted to 20% with sterile saline) for 4 weeks, and vital staining of the ocular surface was compared before and after treatment. The effects of serum on mucin (MUC-1) expression were observed in cultured conjunctival epithelial cells in vitro. RESULTS—EGF, vitamin A, and TGF-β were well preserved for up to 1 month in the refrigerator at 4°C and up to 3 months in the freezer at −20°C. Rose bengal and fluorescein scores improved significantly from the initial scores of 5.3 and 5.6 to 1.7 and 2.5 after 4 weeks, respectively. The additive effect of human serum for cultured conjunctival epithelial cells showed significant MUC-1 upregulation on the cell surface. CONCLUSION—Autologous serum application is a safe and efficient way to provide essential components to the ocular surface in the treatment of dry eye associated with Sjögren's syndrome. Keywords: autologous serum; Sjögren's syndrome; tears ocular surface
Full Text
The Full Text of this article is available as a PDF (182.6 KB).
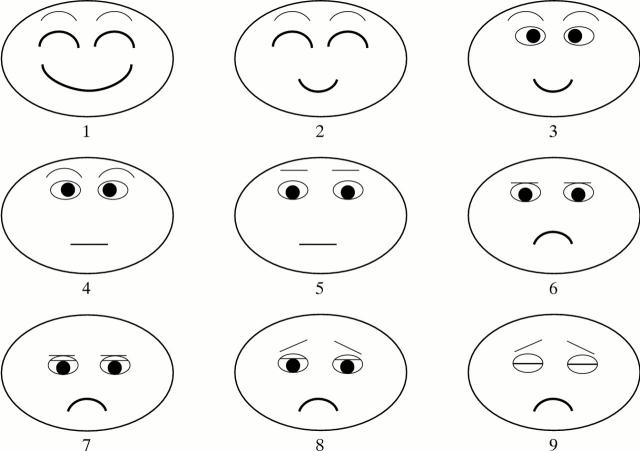
Figure 1 .
Face score, nine different expressions describing the condition of patients' eyes.
Figure 2 .
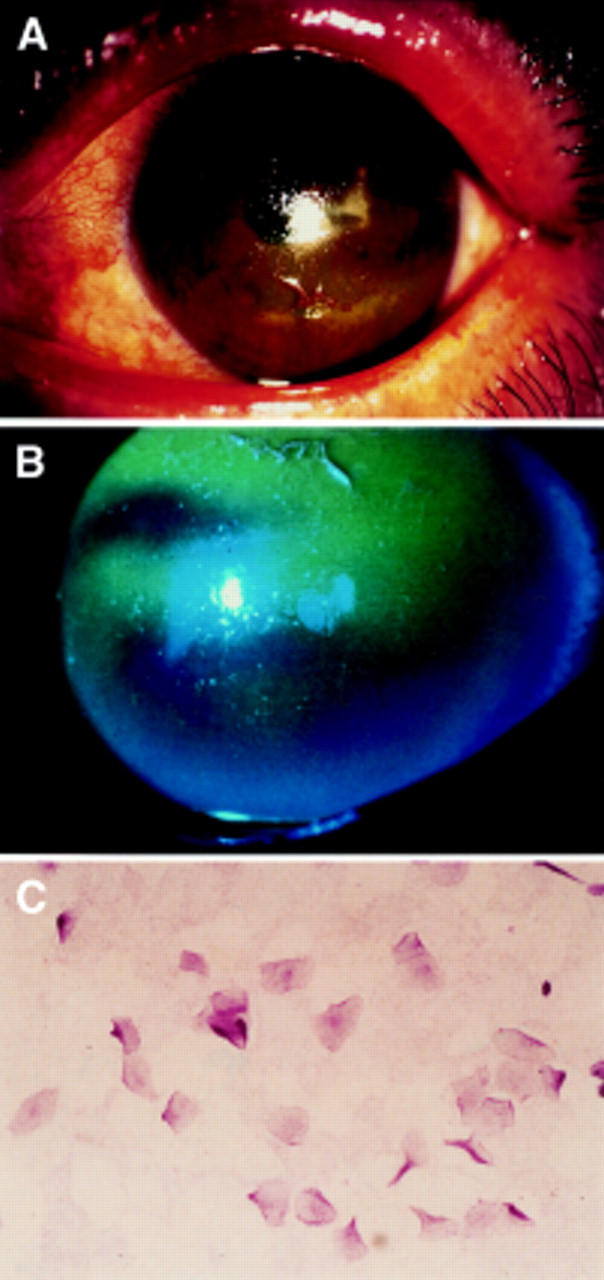
Slit lamp photographs stained by rose bengal and fluorescein of the left eye of the patients described (A, B). Note the corneal epithelium was deeply stained by fluorescein. Impression cytology of the bulbar conjunctiva in patients with Sjögren's syndrome (C) stained by PAS staining. There are no goblet cells observed. The epithelial cells are enlarged showing the squamous metaplasia.
Figure 3 .
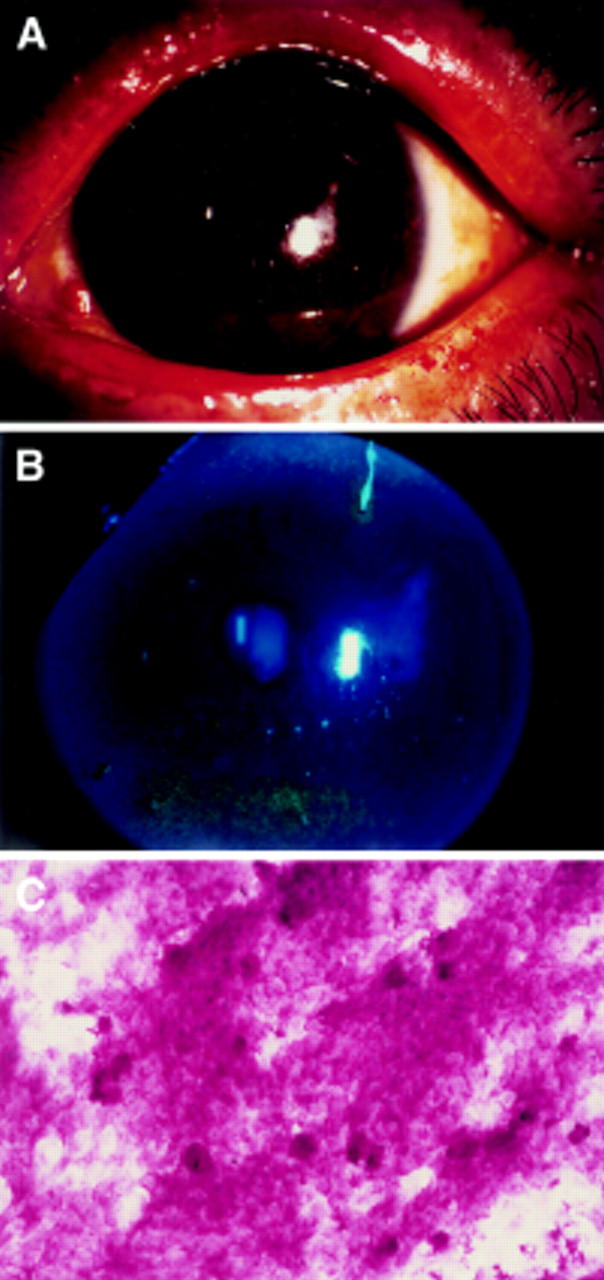
Slit lamp photographs stained by rose bengal and fluorescein of the left eye of the patients after the autologous serum treatment for 1 month. (A, B) Note the corneal epithelium dramatically improved. Impression cytology of the bulbar conjunctiva in patients with Sjögren's syndrome also improved (C). Goblet cells are observed with smaller epithelial cells.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farris R. L. The dry eye: its mechanisms and therapy, with evidence that contact lens is a cause. CLAO J. 1986 Oct-Dec;12(4):234–246. [PubMed] [Google Scholar]
- Fox R. I., Chan R., Michelson J. B., Belmont J. B., Michelson P. E. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984 Apr;27(4):459–461. doi: 10.1002/art.1780270415. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Saito I. Criteria for diagnosis of Sjögren's syndrome. Rheum Dis Clin North Am. 1994 May;20(2):391–407. [PubMed] [Google Scholar]
- Gupta A., Monroy D., Ji Z., Yoshino K., Huang A., Pflugfelder S. C. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996 Jun;15(6):605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- Hamano H., Hori M., Hamano T., Mitsunaga S., Maeshima J., Kojima S., Kawabe H., Hamano T. A new method for measuring tears. CLAO J. 1983 Jul-Sep;9(3):281–289. [PubMed] [Google Scholar]
- Hikichi T., Yoshida A., Tsubota K. Lymphocytic infiltration of the conjunctiva and the salivary gland in Sjögren's syndrome. Arch Ophthalmol. 1993 Jan;111(1):21–22. doi: 10.1001/archopht.1993.01090010023009. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Epidermal growth factor (urogastrone) in human fluids: size heterogeneity. J Clin Endocrinol Metab. 1979 Apr;48(4):673–679. doi: 10.1210/jcem-48-4-673. [DOI] [PubMed] [Google Scholar]
- Lemp M. A. Recent developments in dry eye management. Ophthalmology. 1987 Oct;94(10):1299–1304. doi: 10.1016/s0161-6420(87)80015-5. [DOI] [PubMed] [Google Scholar]
- Lemp M. A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995 Oct;21(4):221–232. [PubMed] [Google Scholar]
- Ohashi Y., Motokura M., Kinoshita Y., Mano T., Watanabe H., Kinoshita S., Manabe R., Oshiden K., Yanaihara C. Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1879–1882. [PubMed] [Google Scholar]
- Pflugfelder S. C., Huang A. J., Feuer W., Chuchovski P. T., Pereira I. C., Tseng S. C. Conjunctival cytologic features of primary Sjögren's syndrome. Ophthalmology. 1990 Aug;97(8):985–991. doi: 10.1016/s0161-6420(90)32478-8. [DOI] [PubMed] [Google Scholar]
- Speek A. J., van Agtmaal E. J., Saowakontha S., Schreurs W. H., van Haeringen N. J. Fluorometric determination of retinol in human tear fluid using high-performance liquid chromatography. Curr Eye Res. 1986 Nov;5(11):841–845. doi: 10.3109/02713688609029235. [DOI] [PubMed] [Google Scholar]
- Toda I., Shimazaki J., Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995 Feb;102(2):302–309. doi: 10.1016/s0161-6420(95)31024-x. [DOI] [PubMed] [Google Scholar]
- Toda I., Tsubota K. Practical double vital staining for ocular surface evaluation. Cornea. 1993 Jul;12(4):366–367. doi: 10.1097/00003226-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Toda I., Yagi Y., Hata S., Itoh S., Tsubota K. Excimer laser photorefractive keratectomy for patients with contact lens intolerance caused by dry eye. Br J Ophthalmol. 1996 Jul;80(7):604–609. doi: 10.1136/bjo.80.7.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Farazdaghi M., Rider A. A. Conjunctival transdifferentiation induced by systemic vitamin A deficiency in vascularized rabbit corneas. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1497–1504. [PubMed] [Google Scholar]
- Tseng S. C. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985 Jun;92(6):728–733. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993 Feb 25;328(8):584–584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol. 1995 Feb;113(2):155–158. doi: 10.1001/archopht.1995.01100020037025. [DOI] [PubMed] [Google Scholar]
- Tsubota K. New approaches to dry-eye therapy. Int Ophthalmol Clin. 1994 Winter;34(1):115–128. doi: 10.1097/00004397-199403410-00011. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Ohyama M., Toda I., Takano Y., Ono M., Shinozaki N., Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996 Jul;122(1):38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Shimazaki J. Treatment of severe dry eye. Lancet. 1996 Jul 13;348(9020):123–123. doi: 10.1016/s0140-6736(96)24028-0. [DOI] [PubMed] [Google Scholar]
- Tsubota K. The importance of the Schirmer test with nasal stimulation. Am J Ophthalmol. 1991 Jan 15;111(1):106–108. doi: 10.1016/s0002-9394(14)76908-9. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Toda I., Nakamori K. Poor illumination, VDTs, and desiccated eyes. Lancet. 1996 Mar 16;347(9003):768–769. doi: 10.1016/s0140-6736(96)90122-1. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Toda I., Yagi Y., Ogawa Y., Ono M., Yoshino K. Three different types of dry eye syndrome. Cornea. 1994 May;13(3):202–209. doi: 10.1097/00003226-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Xu K. P., Fujihara T., Katagiri S., Takeuchi T. Decreased reflex tearing is associated with lymphocytic infiltration in lacrimal glands. J Rheumatol. 1996 Feb;23(2):313–320. [PubMed] [Google Scholar]
- Tsubota K., Yamada M., Urayama K. Spectacle side panels and moist inserts for the treatment of dry-eye patients. Cornea. 1994 May;13(3):197–201. doi: 10.1097/00003226-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Ubels J. L., Foley K. M., Rismondo V. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci. 1986 Aug;27(8):1261–1268. [PubMed] [Google Scholar]
- Wilson S. E. Lacrimal gland epidermal growth factor production and the ocular surface. Am J Ophthalmol. 1991 Jun 15;111(6):763–765. doi: 10.1016/s0002-9394(14)76785-6. [DOI] [PubMed] [Google Scholar]
- Wilson S. E., Lloyd S. A., Kennedy R. H. Basic fibroblast growth factor (FGFb) and epidermal growth factor (EGF) receptor messenger RNA production in human lacrimal gland. Invest Ophthalmol Vis Sci. 1991 Sep;32(10):2816–2820. [PubMed] [Google Scholar]
- Xu K. P., Katagiri S., Takeuchi T., Tsubota K. Biopsy of labial salivary glands and lacrimal glands in the diagnosis of Sjögren's syndrome. J Rheumatol. 1996 Jan;23(1):76–82. [PubMed] [Google Scholar]
- el-Ghorab M., Capone A., Jr, Underwood B. A., Hatchell D., Friend J., Thoft R. A. Response of ocular surface epithelium to corneal wounding in retinol-deficient rabbits. Invest Ophthalmol Vis Sci. 1988 Nov;29(11):1671–1676. [PubMed] [Google Scholar]
- van Setten G. B., Macauley S., Humphreys-Beher M., Chegini N., Schultz G. Detection of transforming growth factor-alpha mRNA and protein in rat lacrimal glands and characterization of transforming growth factor-alpha in human tears. Invest Ophthalmol Vis Sci. 1996 Jan;37(1):166–173. [PubMed] [Google Scholar]
- van Setten G. B., Tervo T., Tervo K., Tarkkanen A. Epidermal growth factor (EGF) in ocular fluids: presence, origin and therapeutical considerations. Acta Ophthalmol Suppl. 1992;(202):54–59. doi: 10.1111/j.1755-3768.1992.tb02169.x. [DOI] [PubMed] [Google Scholar]
- van Setten G. B., Viinikka L., Tervo T., Pesonen K., Tarkkanen A., Perheentupa J. Epidermal growth factor is a constant component of normal human tear fluid. Graefes Arch Clin Exp Ophthalmol. 1989;227(2):184–187. doi: 10.1007/BF02169794. [DOI] [PubMed] [Google Scholar]