Abstract
BACKGROUND/AIM—Nasal administration of retinal antigens induces systemic tolerance which results in suppression of experimental autoimmune uveoretinitis (EAU) when subsequently exposed to antigen. The aim was to establish if tolerance induction alters retinal infiltrating leucocyte phenotype and cytokine profile in tolerised animals when there is significantly reduced tissue destruction despite immunisation with retinal antigen. METHODS—Female Lewis rats were tolerised by intranasal administration with retinal extract (RE) before immunisation with RE to induce EAU. Control animals were administered phosphate buffered saline (PBS) intranasally. Post immunisation, daily clinical responses were recorded and at the height of disease, retinas were removed and either infiltrating leucocytes isolated for flow cytometric phenotype assessment and intracellular cytokine production, or chorioretina processed for immunohistochemistry. Fellow eyes were assessed for cytokine mRNA by semiquantitative RT-PCR. RESULTS—Flow cytometric analysis showed that before clinical onset of EAU there is no evidence of macrophage infiltration and no significant difference in circulating T cell populations within the retina. By day 14 a reduced retinal infiltrate in tolerised animals was observed and in particular a reduction in numbers of "activated" (with respect to CD4 and MHC class II expression) macrophages. Immunohistochemistry confirmed these findings and additionally minimal rod outer segment destruction was observed histologically. Cytokine analysis revealed that both IL-10 mRNA and intracellular IL-10 production was increased in tolerised eyes 7 days post immunisation. Although by day 14 post immunisation, IL-10 production was equivalent in both groups, a reduced percentage of IFN-γ+ macrophages and IFN-γ+ CD4+ T cells with increased percentage of IL-4+ CD4+ T cells were observed in tolerised animals. CONCLUSIONS—Leucocytic infiltrate is not only reduced in number but its distinct phenotype compared with controls implies a reduced activation status of infiltrating monocytes to accompany increased IL-10 and reduced IFN-γ production in tolerised animals. This modulation may in turn contribute towards protection against target organ destruction in EAU. Keywords: intranasal tolerance; experimental autoimmune uveoretinitis; retina; cytokines; flow cytometry
Full Text
The Full Text of this article is available as a PDF (253.8 KB).
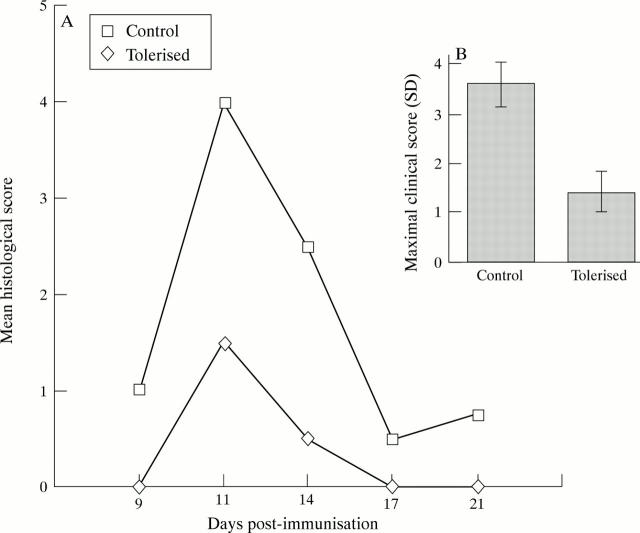
Figure 1 .
Suppression of clinicopathological features of experimental autoimmune uveoretinitis (EAU) by intranasal tolerance induction. (A) Histological grading12 of EAU shows extent of histopathological damage is reduced in tolerised animals. (B) Clinical inflammatory scores12 are significantly suppressed in tolerised animals.
Figure 2 .
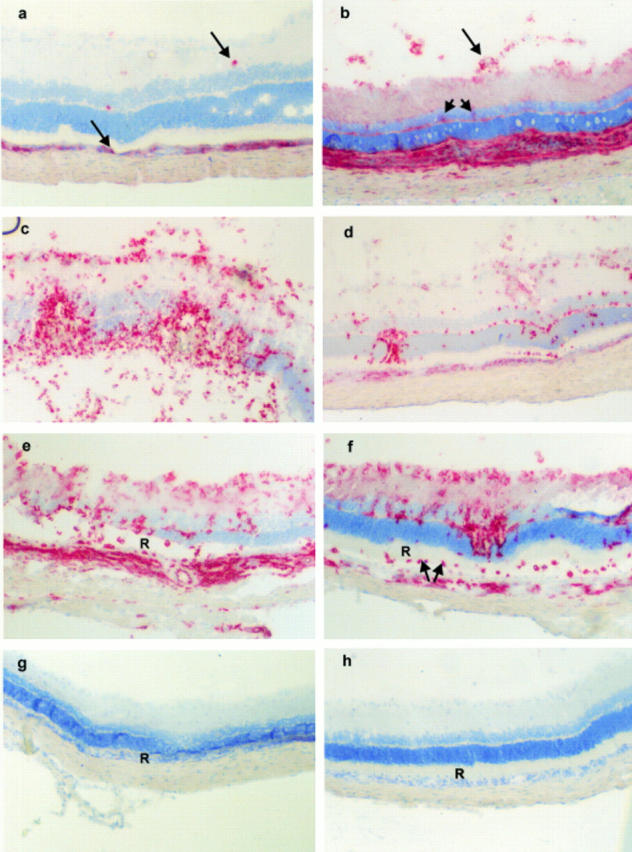
Composite of immunohistochemical analysis of chorioretina from tolerised and control animals. By day 9 post immunisation ED1+ macrophages are found infiltrating both the choroid and retina (arrows) (a), concomitant with an increase in MHC class II staining (b) particularly at the choroid/RPE, inner retinal vessels (arrow), and retinal parenchyma (arrowhead representing parenchymal microglia), both panels are from control eyes. During active inflammation (day 11 post immunisation) ED1+ cell infiltrate increases in number in both control (c) and tolerised animals (d), although staining is more intense in control animals (c). By day 14 post immunisation with persistent ED1+ infiltrate, retinal architecture (particularly ROS (R)) is markedly damaged in control animals (e) whereas tolerised animals display preserved ROS (R) despite persistent ED1+ infiltrate (f). This is further confirmed by day 21 post immunisation. Although leucocytic infiltrate has largely resolved total ROS loss (R) can be observed in retinas of control (g) and not tolerised animals (h). Original magnifications: a-f ×350 and g, h ×300.
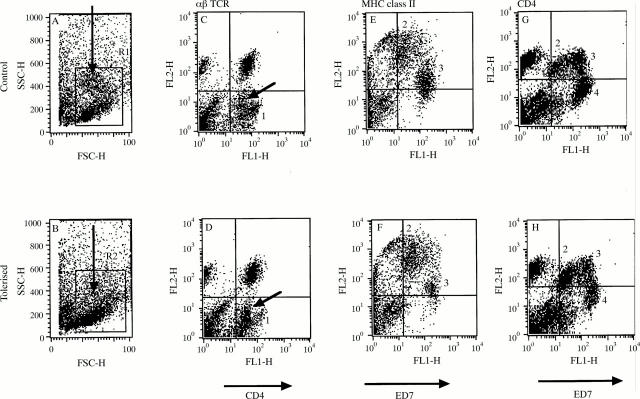
Figure 3 .
Flow cytometric analysis of retinal leucocytes in tolerised and control animals during EAU. Retinal leucocytes were isolated day 14 post immunisation The cells were derived from a pool of four eyes per experimental group, but comparable data were obtained in another identical experiment. Populations identified were based on two colour flow cytometric analysis including CD4 v αβTCR (CA and DC) and ED7 vMHC class II (BE and FD) and ED7 v CD4 (G and H)); see text. Plots A and B show scatter profile of retinal cell isolates demonstrating an increased population of granulocytes (arrow) in control animals (see text). Population 1 (plots C and D) identifies a CD4highαβ TCR- population in control animals (CD4 MFI of 110), distinct from CD4lowαβTCR- (arrow) population (CD4 MFI of 43), representing microglia (MG) and non-activated infiltrating macrophages.22 25 26 MG, further shown (population 2) on plots E-H and characterised by ED7low expression (see text), are equal in number (data not shown) and express similar MHC class II and CD4 between the two groups. Population 3 represent ED7high cells (granulocytes are excluded for calculation of macrophage numbers by appropriate backgating to scatter plot). Increased numbers of macrophages express MHC class II (plots E and F) and high levels of CD4 (plots G and H) in control animals (see text). E and F do not show granulocyte population whereas plots G and H include granulocytes (population 4) to show that they do not express CD4 and are markedly reduced in number in tolerised animals.
Figure 4 .
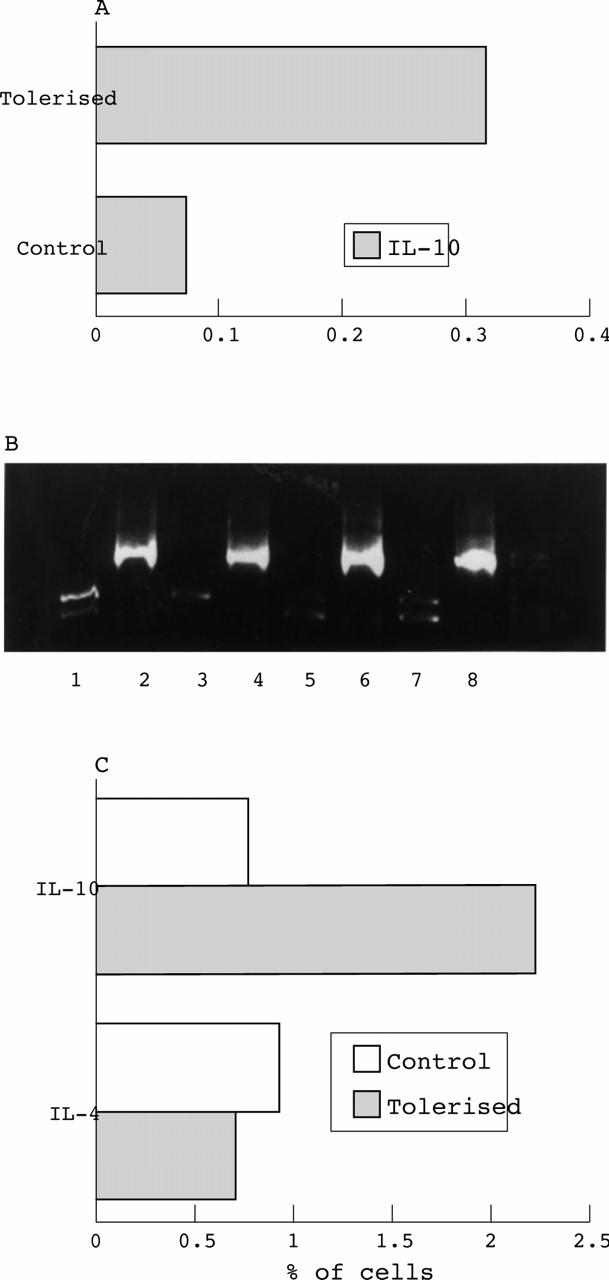
(A) RT-PCR cytokine analysis from eyes of control and tolerised animals during EAU. Values are from a representative experiment calculated from means of two animals/group at day 7 post immunisation each time point. (B) PCR IL-10 blots at day 7 post immunisation. Lanes 1 and 3, tolerised IL-10; lanes 2 and 4, tolerised β actin; lanes 5 and 7, control IL-10; lanes 6 and 8 control β actin. (C) Flow cytometric intracellular cytokine analysis day 7 post immunisation in control and tolerised animals. IL-10 expression represented as percentage of OX1+ cells. Values are mean of two animals/group.
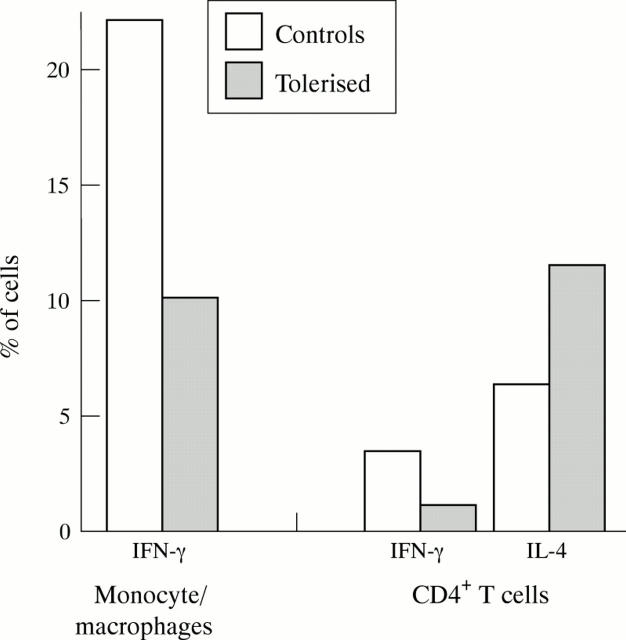
Figure 5 .
Flow cytometric intracellular cytokine analysis day 14 post immunisation in control and tolerised animals. Cytokine expression represented as percentage of either monocyte/macrophage gate or CD4+ T cells. Values are mean of two animals/group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai X. F., Li H. L., Shi F. D., Liu J. Q., Xiao B. G., Van der Meide P. H., Link H. Complexities of applying nasal tolerance induction as a therapy for ongoing relapsing experimental autoimmune encephalomyelitis (EAE) in DA rats. Clin Exp Immunol. 1998 Jan;111(1):205–210. doi: 10.1046/j.1365-2249.1998.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X. F., Shi F. D., Xiao B. G., Li H. L., van der Meide P. H., Link H. Nasal administration of myelin basic protein prevents relapsing experimental autoimmune encephalomyelitis in DA rats by activating regulatory cells expressing IL-4 and TGF-beta mRNA. J Neuroimmunol. 1997 Dec;80(1-2):65–75. doi: 10.1016/s0165-5728(97)00133-1. [DOI] [PubMed] [Google Scholar]
- Barton K., Lightman S. T lymphocyte effector mechanisms in the retina in posterior uveitis. Eye (Lond) 1994;8(Pt 1):60–65. doi: 10.1038/eye.1994.11. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Chan C. C., Fujino Y., Najafian F., Grover S., Hansen C. T., Wilder R. L. Recruitment of antigen-nonspecific cells plays a pivotal role in the pathogenesis of a T cell-mediated organ-specific autoimmune disease, experimental autoimmune uveoretinitis. J Neuroimmunol. 1993 Sep;47(2):177–188. doi: 10.1016/0165-5728(93)90028-w. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chen Y., Inobe J., Kuchroo V. K., Baron J. L., Janeway C. A., Jr, Weiner H. L. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazio T. J., Niederkorn J. Y. A novel role for TGF-beta and IL-10 in the induction of immune privilege. J Immunol. 1998 Mar 1;160(5):2089–2098. [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., Liversidge J., Forrester J. V. Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye (Lond) 1994;8(Pt 1):52–59. doi: 10.1038/eye.1994.10. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., Liversidge J., Forrester J. V. Intranasal administration of retinal antigens suppresses retinal antigen-induced experimental autoimmune uveoretinitis. Immunology. 1994 Aug;82(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., McKinnon A., Liversidge J., Forrester J. V. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993 Mar;77(3):171–175. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A. D., Duncan L., Hale G., Waldmann H., Isaacs J. Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998 Jun;11(3):255–264. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Kreutzer B., Laliotou B., Forrester J. V. Effects of mycophenolate mofetil on nasal mucosal tolerance induction. Invest Ophthalmol Vis Sci. 1998 Apr;39(5):835–840. [PubMed] [Google Scholar]
- Dick A. D., McMenamin P. G., Körner H., Scallon B. J., Ghrayeb J., Forrester J. V., Sedgwick J. D. Inhibition of tumor necrosis factor activity minimizes target organ damage in experimental autoimmune uveoretinitis despite quantitatively normal activated T cell traffic to the retina. Eur J Immunol. 1996 May;26(5):1018–1025. doi: 10.1002/eji.1830260510. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Huitinga I., Lumsden L., Dijkstra C. D. Marrow-derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr Eye Res. 1998 Apr;17(4):426–437. doi: 10.1080/02713689808951224. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Liversidge J., Dua H. S., Towler H., McMenamin P. G. Comparison of clinical and experimental uveitis. Curr Eye Res. 1990;9 (Suppl):75–84. doi: 10.3109/02713689008999424. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Donoso L. A. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993 Nov 15;151(10):5751–5761. [PubMed] [Google Scholar]
- Hoey S., Grabowski P. S., Ralston S. H., Forrester J. V., Liversidge J. Nitric oxide accelerates the onset and increases the severity of experimental autoimmune uveoretinitis through an IFN-gamma-dependent mechanism. J Immunol. 1997 Nov 15;159(10):5132–5142. [PubMed] [Google Scholar]
- Huitinga I., van Rooijen N., de Groot C. J., Uitdehaag B. M., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990 Oct 1;172(4):1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer B., Laliotou B., Cheng Y. F., Liversidge J., Forrester J. V., Dick A. D. Nasal administration of retinal antigens maintains immunosuppression of uveoretinitis in cyclosporin-A-treated Lewis rats: future treatment of endogenous posterior uveoretinitis? Eye (Lond) 1997;11(Pt 4):445–452. doi: 10.1038/eye.1997.125. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Metzler B., Wraith D. C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993 Sep;5(9):1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- Myers L. K., Seyer J. M., Stuart J. M., Kang A. H. Suppression of murine collagen-induced arthritis by nasal administration of collagen. Immunology. 1997 Feb;90(2):161–164. doi: 10.1046/j.1365-2567.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B., Caspi R. R., Mahdi R., Chan C. C., Roberge F., Lider O., Weiner H. L. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral induction of tolerance with S-antigen. J Immunol. 1990 Mar 1;144(5):1689–1695. [PubMed] [Google Scholar]
- Nussenblatt R. B., Gery I., Weiner H. L., Ferris F. L., Shiloach J., Remaley N., Perry C., Caspi R. R., Hafler D. A., Foster C. S. Treatment of uveitis by oral administration of retinal antigens: results of a phase I/II randomized masked trial. Am J Ophthalmol. 1997 May;123(5):583–592. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C., Metcalfe D. D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995 Dec 15;188(1):117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Roper G. J., Kaplan H. J. Presumed ocular histoplasmosis syndrome in The Netherlands. Br J Ophthalmol. 1997 Jan;81(1):1–1. doi: 10.1136/bjo.81.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F. D., Bai X. F., Li H. L., Huang Y. M., Van der Meide P. H., Link H. Nasal tolerance in experimental autoimmune myasthenia gravis (EAMG): induction of protective tolerance in primed animals. Clin Exp Immunol. 1998 Mar;111(3):506–512. doi: 10.1046/j.1365-2249.1998.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurau S. R., Chan C. C., Nussenblatt R. B., Caspi R. R. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997 Aug;109(2):370–376. doi: 10.1046/j.1365-2249.1997.4571356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Atkinson M. A., Clare-Salzler M., Herschenfeld A., Forsthuber T., Lehmann P. V., Kaufman D. L. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996 Apr 1;183(4):1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvers D. A., van der Cammen M. J., Kraal G. Mucosal tolerance is associated with, but independent of, up-regulation Th2 responses. Immunology. 1997 Nov;92(3):328–333. doi: 10.1046/j.1365-2567.1997.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsukura J., Huang H., Singh A. K., Shichi H. Regulatory cells generated by testicular tolerization to retinal S-antigen: possible involvement of IL-4, IL-10, and TGF-beta in the suppression of experimental autoimmune uveoretinitis. Cell Immunol. 1997 Dec 15;182(2):89–98. doi: 10.1006/cimm.1997.1229. [DOI] [PubMed] [Google Scholar]