Abstract
AIMS—To investigate the distribution, persistence, and stability of fluorescently labelled phosphorothioate oligonucleotides (PS-ODNs) in normal and laser photocoagulated retina following intravitreal injection in the rat. METHODS—Fluorescently labelled PS-ODNs were injected intravitreally into pigmented eyes at doses of 0.5-10.0 nmol in 2.0 µl solution. The dynamics of PS-ODNs was evaluated by fluorescent microscopy of cryosections and flat mounted retinal pigment epithelium (RPE)-choroid-sclera. Genescan analysis was used to assess the integrity of PS-ODNs in the retina after injection. The dynamics of PS-ODNs was also evaluated in the retina following krypton laser photocoagulation with a protocol producing choroidal neovascularisation (CNV). RESULTS—Following intravitreal injection the PS-ODNs demonstrated dose and time dependent distribution and persistence in the retina, where they accessed all neural layers. However, they preferentially accumulated in the RPE layer, demonstrated as bright granules in the cytoplasm of the cells. Injections of 5.0 and 7.5 nmol of PS-ODNs exhibited strong fluorescence in the retina for 6 weeks after injection. Genescan analysis demonstrated that the PS-ODNs remained almost completely intact for at least 12 weeks. Following laser treatment, the PS-ODNs were concentrated in the regions of laser photocoagulation and retained high intensity for at least 8 weeks after injection, particularly localised to macrophages, RPE, and the local choroidal tissue. CONCLUSIONS—These results indicate that PS-ODNs are stable and accessible to most neural layers of the retina, and they preferentially accumulate in the RPE layer following intravitreal injection. The successful delivery of PS-ODNs into normal and laser photocoagulated retina suggests that PS-ODNs may have potential in the development of therapy for attenuating retinal degenerations and CNV.
Full Text
The Full Text of this article is available as a PDF (275.9 KB).
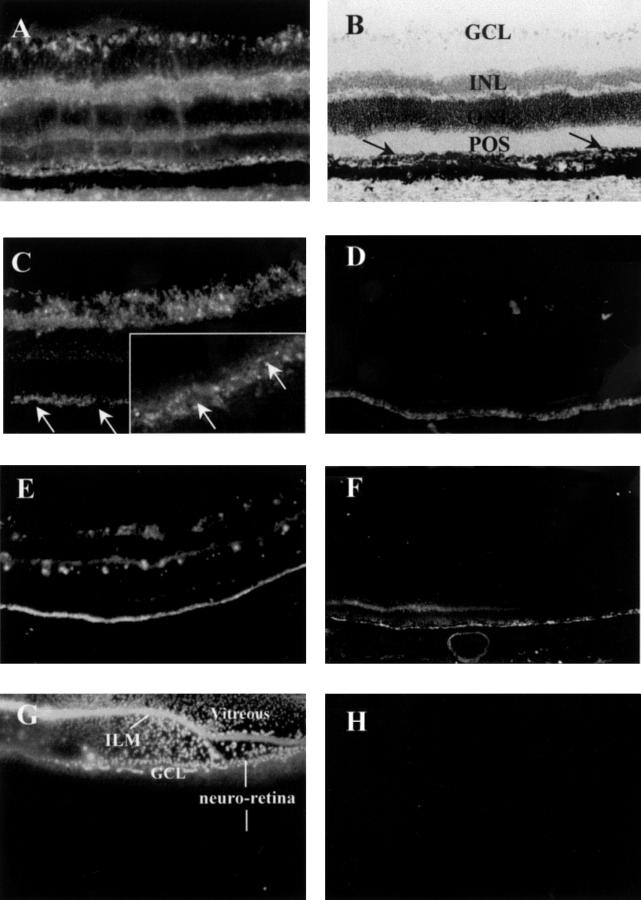
Figure 1 .
Distribution and persistence of PS-ODNs (DS 012) in the retina 2 weeks after intravitreal injection (A-D). (A) 10.0 nmol, (B) retinal morphology of (A), counterstaining with haemotoxylin. The arrows indicate the RPE layer. (C) 2.0 nmol, the arrows are pointing to the nuclei of RPE cells. Inset: cytoplasmic distribution of PS-ODN in RPE observed by confocal laser scanning microscopy. (D) 0.5 nmol. (E and F) 5.0 nmol, 6 weeks (E) and 8 weeks (F) after injection. (G and H) Fluorescent microscopic images from the control eyes injected with fluorescent 6-FAM at 1 hour (G) and 24 (H) hours after injection. The fluorescent 6-FAM was mostly confined to the vitreous but some just entered into the ganglion cell layer at 1 hour after injection (G). However, no fluorescent signal was detected in the neural retina at 24 hours after injection (H). Original magnifications: (A-H) ×50; inset of (C) ×100. RPE = retinal pigment epithelium; POS = photoreceptor outer segment; ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer; ILM = inner limiting membrane.
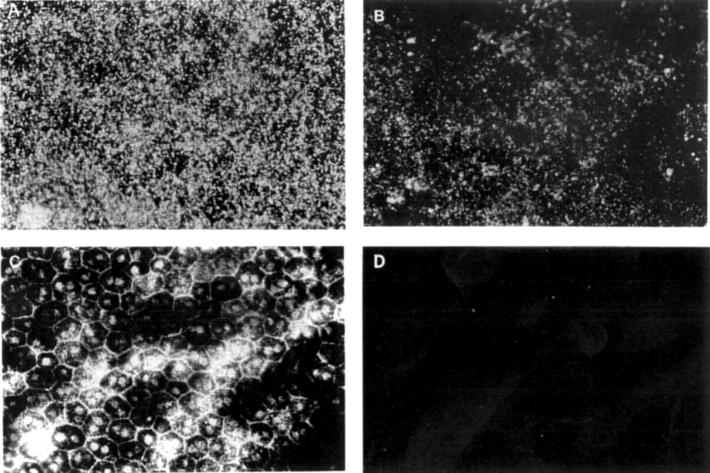
Figure 2 .
Distribution of fluorescently labelled PS-ODN (DS 012, 5.0 nmol) in the RPE cells by fluorescent microscopy of flat mounted RPE-choroid-sclera. (A) Two weeks after injection, the fluorescently labelled PS-ODNs appear as bright granules in the cytoplasm of the RPE cells. (B) Eight weeks after injection. (C) Microphotograph of the hexagonal RPE cells in (A), observed by light microscopy. (D) A control eye injected with 5.0 nmol of sodium fluorescein, 2 week after injection. Original magnification (A-D) ×100.
Figure 3 .
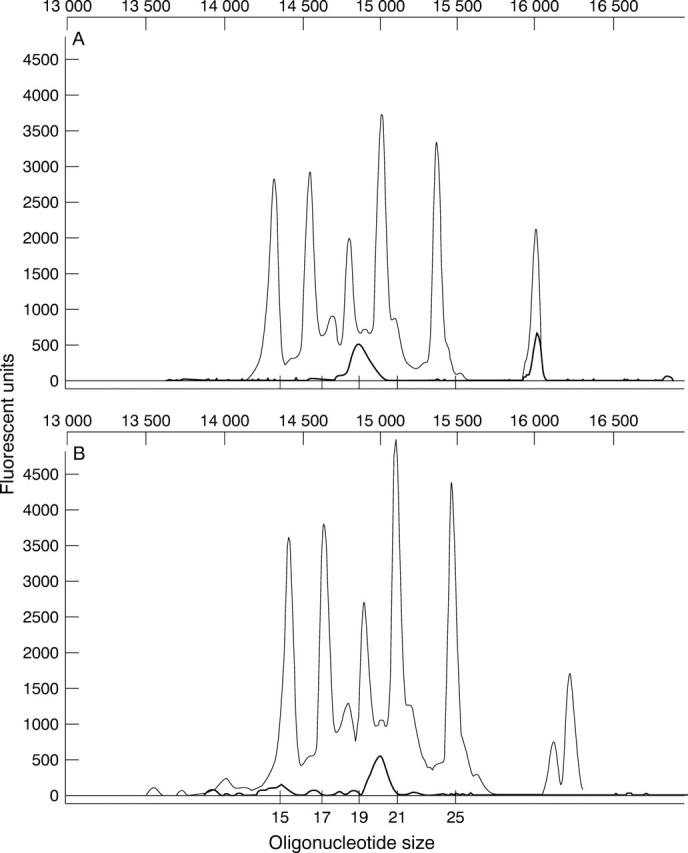
Accurate Genescan sizing of injected and uninjected PS-ODN using oligonucleotide size standards. (A) The original 20-mer DS 012 was compared directly with a series of PS-ODNs varying in size from 15 to 25 nucleotides in length and their electrophoretograms superimposed using the internal standard as a reference. Oligonucleotides within this range of size were clearly resolved using the Genescan analysis software under the altered run conditions. (B) DNA extracted from the retina of an eye 12 weeks following injection with DS 012 for 12 weeks was run and compared directly with the oligonucleotide size standard. The extracted oligonucleotide demonstrated some minor peaks representing N-1 degradation products.
Figure 4 .
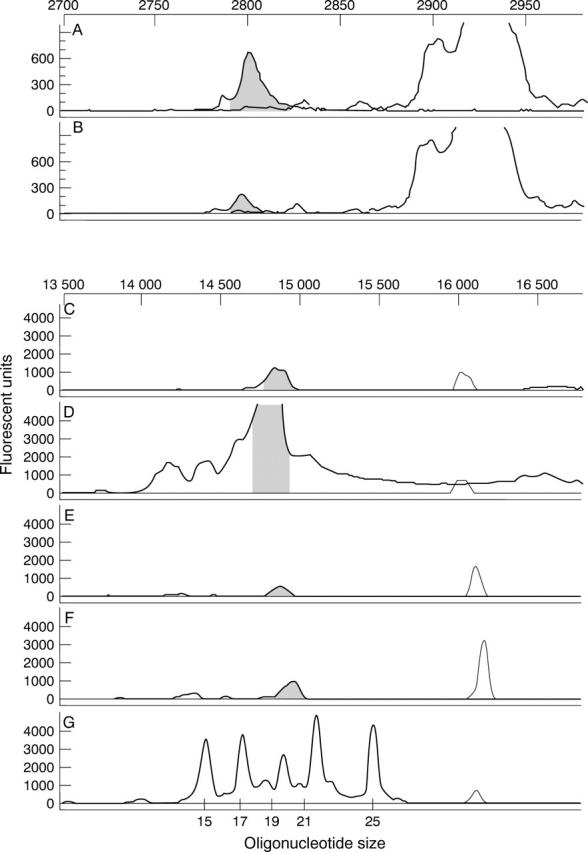
Persistence of PS-ODN following intravitreal injection demonstrated by Genescan analysis. Electrophoretograms of DNA extracted from the retina (A, C, and E) and the RPE-choroid-sclera complex (B, D, and F) at 6 weeks (A, B), 8 weeks (C, D), and 12 weeks (E, F) after injection with DS 012, and the oligonucleotide size standard (G). The internal standard is superimposed in all electrophoretograms at the same data collection point and the peaks for the PS-ODN are equivalent for both tissue samples at all time points. Note the actual fluorescent intensity of 6 week samples is effectively 130 times higher than that of 8 and 12 week samples.
Figure 5 .
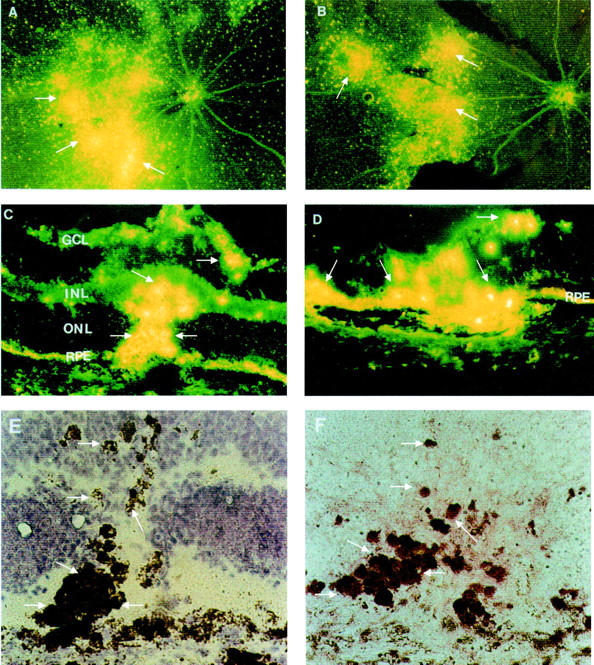
Distribution of PS-ODNs (DS 012, 5.0 nmol) in the retina following krypton laser photocoagulation. (A, B) Retinal whole mounts, 4 weeks (A) and 8 weeks (B) after injection; the PS-ODNs are concentrated in the regions of laser photocoagulation with an appearance of clumps of bright granules at the sites of laser burns (arrows). (C, D) Cryosections from eyes injected with DS 012 following krypton laser photocoagulation, 4 weeks (C) and 8 weeks (D) after injection. The fluorescent signal is particularly localised to infiltrating cells (arrows) and the RPE layer. (E) Higher magnification of (C) observed by light microscopy, counterstaining with haemotoxylin, the arrows indicating pigment laden infiltrating cells. (F) A serial section of (C) and (E), immunostaining for CD68 (arrows). Original magnification: (A, B) ×10; (C, D) ×50; (E, F) ×100. GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer; RPE = retinal pigment epithelium.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Iyer R. P. Modified oligonucleotides as therapeutic and diagnostic agents. Curr Opin Biotechnol. 1995 Feb;6(1):12–19. doi: 10.1016/0958-1669(95)80003-4. [DOI] [PubMed] [Google Scholar]
- Bandello F., Brancato R., Trabucchi G., Lattanzio R., Malegori A. Diode versus argon-green laser panretinal photocoagulation in proliferative diabetic retinopathy: a randomized study in 44 eyes with a long follow-up time. Graefes Arch Clin Exp Ophthalmol. 1993 Sep;231(9):491–494. doi: 10.1007/BF00921112. [DOI] [PubMed] [Google Scholar]
- Benimetskaya L., Loike J. D., Khaled Z., Loike G., Silverstein S. C., Cao L., el Khoury J., Cai T. Q., Stein C. A. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997 Apr;3(4):414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- Bressler N. M., Bressler S. B., Fine S. L. Age-related macular degeneration. Surv Ophthalmol. 1988 May-Jun;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Broaddus W. C., Prabhu S. S., Gillies G. T., Neal J., Conrad W. S., Chen Z. J., Fillmore H., Young H. F. Distribution and stability of antisense phosphorothioate oligonucleotides in rodent brain following direct intraparenchymal controlled-rate infusion. J Neurosurg. 1998 Apr;88(4):734–742. doi: 10.3171/jns.1998.88.4.0734. [DOI] [PubMed] [Google Scholar]
- Campbell J. M., Bacon T. A., Wickstrom E. Oligodeoxynucleoside phosphorothioate stability in subcellular extracts, culture media, sera and cerebrospinal fluid. J Biochem Biophys Methods. 1990 Mar;20(3):259–267. doi: 10.1016/0165-022x(90)90084-p. [DOI] [PubMed] [Google Scholar]
- Crooke R. M. In vitro toxicology and pharmacokinetics of antisense oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):609–646. [PubMed] [Google Scholar]
- Dastgheib K., Bressler S. B., Green W. R. Clinicopathologic correlation of laser lesion expansion after treatment of choroidal neovascularization. Retina. 1993;13(4):345–352. doi: 10.1097/00006982-199313040-00013. [DOI] [PubMed] [Google Scholar]
- Eder P. S., DeVine R. J., Dagle J. M., Walder J. A. Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3' exonuclease in plasma. Antisense Res Dev. 1991 Summer;1(2):141–151. doi: 10.1089/ard.1991.1.141. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L., Hilderbrand E. S., Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984 Feb;25(2):195–200. [PubMed] [Google Scholar]
- Finnemann S. C., Bonilha V. L., Marmorstein A. D., Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Aguilar M., Besen G., Vuong C., Tatebayashi M., Munguia D., Gangan P., Wiley C. A., Freeman W. R. Evaluation of retinal toxicity and efficacy of anti-cytomegalovirus and anti-herpes simplex virus antiviral phosphorothioate oligonucleotides ISIS 2922 and ISIS 4015. J Infect Dis. 1997 Jun;175(6):1308–1316. doi: 10.1086/516461. [DOI] [PubMed] [Google Scholar]
- Hangai M., Tanihara H., Honda Y., Kaneda Y. In vivo delivery of phosphorothioate oligonucleotides into murine retina. Arch Ophthalmol. 1998 Mar;116(3):342–348. doi: 10.1001/archopht.116.3.342. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Hata Y., Yoshikawa H., Nakagawa K., Sueishi K., Inomata H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1997 Mar;235(3):159–167. doi: 10.1007/BF00941723. [DOI] [PubMed] [Google Scholar]
- Kennedy C. J., Rakoczy P. E., Constable I. J. Lipofuscin of the retinal pigment epithelium: a review. Eye (Lond) 1995;9(Pt 6):763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- Kliffen M., Sharma H. S., Mooy C. M., Kerkvliet S., de Jong P. T. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997 Feb;81(2):154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa S., Katai N., Shibuki H., Kurokawa T., Umihira J., Nikaido T., Kametani K., Yoshimura N. Expression of cell cycle-related genes in dying cells in retinal ischemic injury. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):610–617. [PubMed] [Google Scholar]
- Leeds J. M., Henry S. P., Bistner S., Scherrill S., Williams K., Levin A. A. Pharmacokinetics of an antisense oligonucleotide injected intravitreally in monkeys. Drug Metab Dispos. 1998 Jul;26(7):670–675. [PubMed] [Google Scholar]
- Leeds J. M., Henry S. P., Truong L., Zutshi A., Levin A. A., Kornbrust D. Pharmacokinetics of a potential human cytomegalovirus therapeutic, a phosphorothioate oligonucleotide, after intravitreal injection in the rabbit. Drug Metab Dispos. 1997 Aug;25(8):921–926. [PubMed] [Google Scholar]
- Lopez P. F., Sippy B. D., Lambert H. M., Thach A. B., Hinton D. R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):855–868. [PubMed] [Google Scholar]
- Lu X. C., Tortella F. C., Ved H. S., Garcia G. E., Dave J. R. Neuroprotective role of c-fos antisense oligonucleotide: in vitro and in vivo studies. Neuroreport. 1997 Sep 8;8(13):2925–2929. doi: 10.1097/00001756-199709080-00024. [DOI] [PubMed] [Google Scholar]
- McCarthy M. M., Masters D. B., Rimvall K., Schwartz-Giblin S., Pfaff D. W. Intracerebral administration of antisense oligodeoxynucleotides to GAD65 and GAD67 mRNAs modulate reproductive behavior in the female rat. Brain Res. 1994 Feb 14;636(2):209–220. doi: 10.1016/0006-8993(94)91019-7. [DOI] [PubMed] [Google Scholar]
- Nickells R. W. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma. 1996 Oct;5(5):345–356. [PubMed] [Google Scholar]
- Ono Y., Watanabe M., Inoue Y., Ohmoto T., Akiyama K., Tsutsui K., Seki S. Developmental expression of APEX nuclease, a multifunctional DNA repair enzyme, in mouse brains. Brain Res Dev Brain Res. 1995 May 26;86(1-2):1–6. doi: 10.1016/0165-3806(94)00212-i. [DOI] [PubMed] [Google Scholar]
- Pizzi M., Valerio A., Ribola M., Spano P. F., Memo M. A Tau antisense oligonucleotide decreases neurone sensitivity to excitotoxic injury. Neuroreport. 1993 Jun;4(6):823–826. doi: 10.1097/00001756-199306000-00057. [DOI] [PubMed] [Google Scholar]
- Putnam D. A. Antisense strategies and therapeutic applications. Am J Health Syst Pharm. 1996 Jan 15;53(2):151–183. doi: 10.1093/ajhp/53.2.151. [DOI] [PubMed] [Google Scholar]
- Rakoczy P. E., Baines M., Kennedy C. J., Constable I. J. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp Eye Res. 1996 Aug;63(2):159–167. doi: 10.1006/exer.1996.0104. [DOI] [PubMed] [Google Scholar]
- Rakoczy P. E., Lai C. M., Baines M., Di Grandi S., Fitton J. H., Constable I. J. Modulation of cathepsin D activity in retinal pigment epithelial cells. Biochem J. 1997 Jun 15;324(Pt 3):935–940. doi: 10.1042/bj3240935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy P. E., Lai M. C., Vijayasekaran S., Robertson T., Rapp L., Papadimitriou J., Constable I. Initiation of impaired outer segment degradation in vivo using an antisense oligonucleotide. Curr Eye Res. 1996 Jan;15(1):119–123. doi: 10.3109/02713689609017619. [DOI] [PubMed] [Google Scholar]
- Rakoczy P. E., Lai M. C., Watson M., Seydel U., Constable I. Targeted delivery of an antisense oligonucleotide in the retina: uptake, distribution, stability, and effect. Antisense Nucleic Acid Drug Dev. 1996 Fall;6(3):207–213. doi: 10.1089/oli.1.1996.6.207. [DOI] [PubMed] [Google Scholar]
- Rakoczy P. E., Mann K., Cavaney D. M., Robertson T., Papadimitreou J., Constable I. J. Detection and possible functions of a cysteine protease involved in digestion of rod outer segments by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994 Nov;35(12):4100–4108. [PubMed] [Google Scholar]
- Reyderman L., Stavchansky S. Pharmacokinetics and biodistribution of a nucleotide-based thrombin inhibitor in rats. Pharm Res. 1998 Jun;15(6):904–910. doi: 10.1023/a:1011980716659. [DOI] [PubMed] [Google Scholar]
- Robinson G. S., Pierce E. A., Rook S. L., Foley E., Webb R., Smith L. E. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C., Price M. T., Almli T., Olney J. W. Excitotoxic neurodegeneration induced by deprivation of oxygen and glucose in isolated retina. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):416–423. [PubMed] [Google Scholar]
- Rosenbaum D. M., Rosenbaum P. S., Gupta A., Michaelson M. D., Hall D. H., Kessler J. A. Retinal ischemia leads to apoptosis which is ameliorated by aurintricarboxylic acid. Vision Res. 1997 Dec;37(24):3445–3451. doi: 10.1016/S0042-6989(96)00328-8. [DOI] [PubMed] [Google Scholar]
- Shen W. Y., Yu M. J., Barry C. J., Constable I. J., Rakoczy P. E. Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat. Br J Ophthalmol. 1998 Sep;82(9):1063–1071. doi: 10.1136/bjo.82.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Y., Rosner M., Turetz J., Belkin M. MK-801 has neuroprotective and antiproliferative effects in retinal laser injury. Invest Ophthalmol Vis Sci. 1997 Jun;38(7):1380–1389. [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A., Kaczmarek L. Antisense oligodeoxyribonucleotides: stability and distribution after intracerebral injection into rat brain. J Neurosci Methods. 1995 Aug;60(1-2):181–187. doi: 10.1016/0165-0270(95)00010-r. [DOI] [PubMed] [Google Scholar]
- Wang Y., Becker D. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med. 1997 Aug;3(8):887–893. doi: 10.1038/nm0897-887. [DOI] [PubMed] [Google Scholar]
- Wong P. Apoptosis, retinitis pigmentosa, and degeneration. Biochem Cell Biol. 1994 Nov-Dec;72(11-12):489–498. doi: 10.1139/o94-066. [DOI] [PubMed] [Google Scholar]
- Yi X., Ogata N., Komada M., Yamamoto C., Takahashi K., Omori K., Uyama M. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch Clin Exp Ophthalmol. 1997 May;235(5):313–319. doi: 10.1007/BF01739641. [DOI] [PubMed] [Google Scholar]
- Young R. W. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987 Mar-Apr;31(5):291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- Zapata A., Capdevila J. L., Tarrason G., Adan J., Martínez J. M., Piulats J., Trullas R. Effects of NMDA-R1 antisense oligodeoxynucleotide administration: behavioral and radioligand binding studies. Brain Res. 1997 Jan 16;745(1-2):114–120. doi: 10.1016/s0006-8993(96)01134-1. [DOI] [PubMed] [Google Scholar]