Abstract
BACKGROUND/AIMS—Although human ocular toxocariasis causes severe vision defect, little is known about its aetiology, diagnosis, and treatment. To develop a new animal model for human ocular toxocariasis, ophthalmological findings of fundi in Mongolian gerbils, Meriones unguiculatus, and BALB/c mice were investigated following infection with Toxocara canis. METHODS—Using an ophthalmoscope, which was specifically developed to observe the fundi of small animals, ocular changes of fundi of 20 gerbils and 11 mice were monitored after oral infection with embryonated eggs of T canis. RESULTS—Vitreous, choroidal, and retinal haemorrhages were consistently observed in Mongolian gerbils, but rarely in mice. Severe exudative lesions and vasculitis were often present in gerbils but not in mice. Migrating larvae were also frequently observed in gerbils. CONCLUSION—Mongolian gerbils are more appropriate animal model for human ocular toxocariasis than previously used experimental animal such as mice, guinea pigs, rabbits, and monkeys because of its high susceptibility of ocular infection.
Full Text
The Full Text of this article is available as a PDF (133.2 KB).
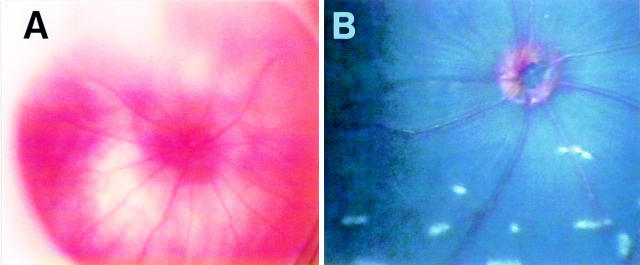
Figure 1 .
Normal fundus of BALB/c mouse and Mongolian gerbils. (A) Normal fundus of BALB/c mouse. (B) Normal fundus of Mongolian gerbils. Rod-shaped multiple white spots were seen in normal fundus of gerbils.
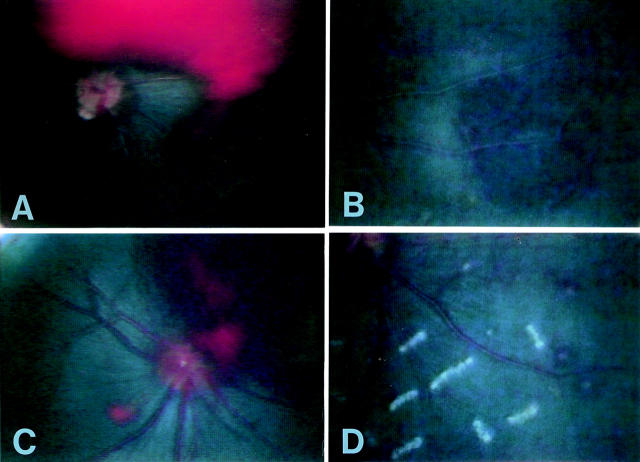
Figure 2 .
Haemorrhagic changes. (A) Vitreous haemorrhage—was seen in two of the 20 gerbils from day 7 after infection of T canis. This was associated with vitreous opacity and was absorbed within 1 month. (B) Choroidal haemorrhage—was found in 95% of gerbils with a single oral inoculation of T canis embryonated eggs. (C) Retinal haemorrhage—was seen in 55% of T canis infected gerbils. Large central retinal haemorrhage was observed. (D) White centred retinal haemorrhage. Multiple small haemorrhage with a white central part were found in the periphery.
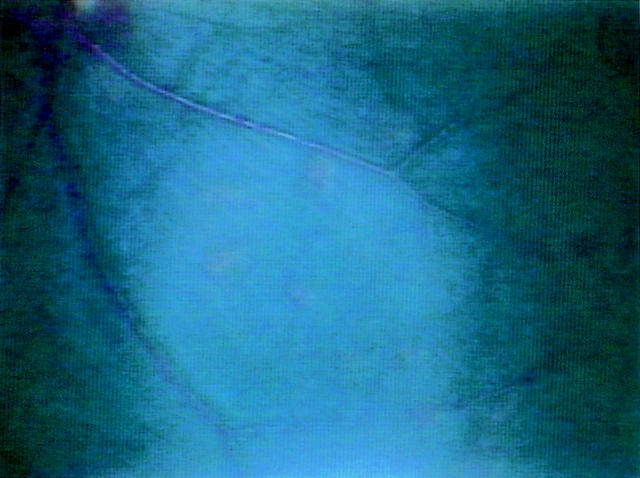
Figure 3 .
Exudative lesion. Irregular colour changes of retinal pattern and soft or hard patches suggested chorioretinitis in 70% of gerbils with oral inoculation of T canis.
Figure 4 .
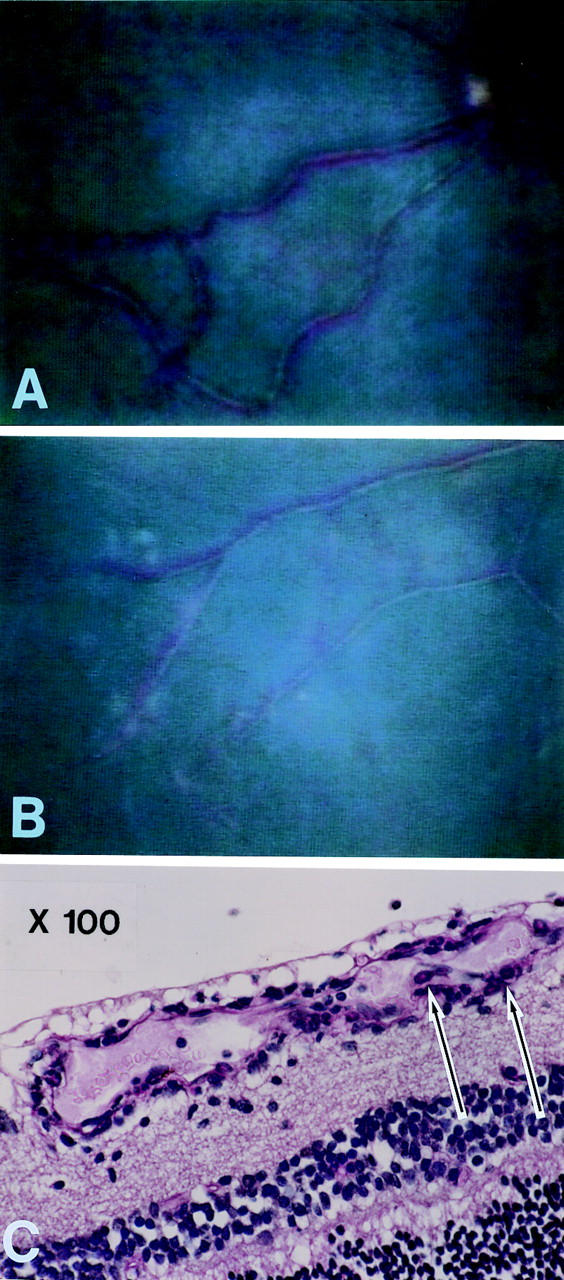
Vasculitis. (A) Tortuosity, dilatation, and narrowing of vessels. (B) White exudative lesion around the bifurcation of vessels. (C) Eosinophils (arrows) and lymphocyte infiltration were seen on the vessel wall.
Figure 5 .
Motile larvae of T canis. Larva (arrow) can migrate vigorously in the retinal layer. Larvae could be found in 80% of gerbils.
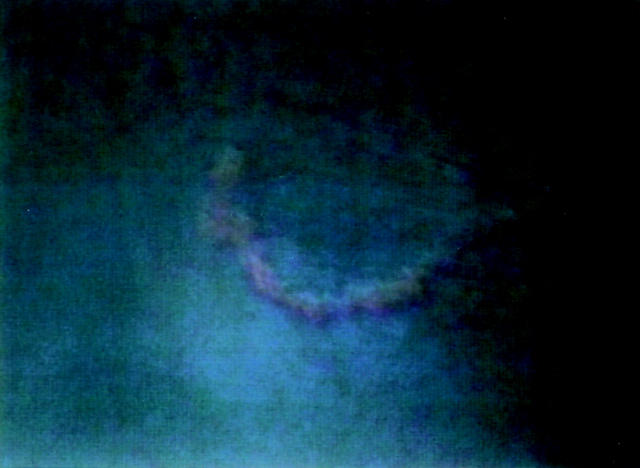
Figure 6 .
Trace of larvae of T canis. This trace disappeared within 20 days.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N. Larval granulomatosis of the retina due to Toxocara. Br J Ophthalmol. 1960 Mar;44:129–148. doi: 10.1136/bjo.44.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont J. B., Irvine A., Benson W., O'Connor G. R. Vitrectomy in ocular toxocariasis. Arch Ophthalmol. 1982 Dec;100(12):1912–1915. doi: 10.1001/archopht.1982.01030040892004. [DOI] [PubMed] [Google Scholar]
- Benitez del Castillo J. M., Herreros G., Guillen J. L., Fenoy S., Bañares A., Garcia J. Bilateral ocular toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol. 1995 Apr;119(4):514–516. doi: 10.1016/s0002-9394(14)71241-3. [DOI] [PubMed] [Google Scholar]
- Brasseur G., Charlin J. F., Brasseur P., Langlois J. Toxocarose oculaire. Acquisitions diagnostiques et thérapeutiques. J Fr Ophtalmol. 1984;7(3):221–226. [PubMed] [Google Scholar]
- Byers B., Kimura S. J. Uveitis after death of a larva in the vitreous cavity. Am J Ophthalmol. 1974 Jan;77(1):63–66. doi: 10.1016/0002-9394(74)90606-0. [DOI] [PubMed] [Google Scholar]
- Campbell D., Chadee K. Interleukin (IL)-2, IL-4, and tumor necrosis factor-alpha responses during Entamoeba histolytica liver abscess development in gerbils. J Infect Dis. 1997 May;175(5):1176–1183. doi: 10.1086/520355. [DOI] [PubMed] [Google Scholar]
- Fitzgerald C. R., Rubin M. L. Intraocular parasite destroyed by photocoagulation. Arch Ophthalmol. 1974 Feb;91(2):162–164. doi: 10.1001/archopht.1974.03900060168015. [DOI] [PubMed] [Google Scholar]
- Gass J. D., Braunstein R. A. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol. 1983 Nov;101(11):1689–1697. doi: 10.1001/archopht.1983.01040020691004. [DOI] [PubMed] [Google Scholar]
- Ghafoor S. Y., Smith H. V., Lee W. R., Quinn R., Girdwood R. W. Experimental ocular toxocariasis: a mouse model. Br J Ophthalmol. 1984 Feb;68(2):89–96. doi: 10.1136/bjo.68.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S. H., Dinning W. J., Voller A., Crowcroft N. S. The spectrum of ocular toxocariasis. Eye (Lond) 1993;7(Pt 3):415–418. doi: 10.1038/eye.1993.82. [DOI] [PubMed] [Google Scholar]
- Glickman L. T., Schantz P. M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- Glickman L., Cypess R., Hiles D., Gessner T. Toxocara-specific antibody in the serum and aqueous humor of a patient with presumed ocular and visceral toxocariasis. Am J Trop Med Hyg. 1979 Jan;28(1):29–35. doi: 10.4269/ajtmh.1979.28.29. [DOI] [PubMed] [Google Scholar]
- Harris W. PSEUDO-GLIOMA DUE TO LARVAL CHOROIDO-RETINAL GRANULOMATOSIS. Br J Ophthalmol. 1961 Feb;45(2):144–146. doi: 10.1136/bjo.45.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVINE W. C., IRVINE A. R., Jr Nematode endophthalmitis: Toxocara canis: report of one case. Am J Ophthalmol. 1959 May;47(5 Pt 2):185–191. doi: 10.1016/s0002-9394(14)78242-x. [DOI] [PubMed] [Google Scholar]
- John T., Donnelly J. J., Rockey J. H. Experimental ocular Toxocara canis and Ascaris suum infection: in vivo and in vitro study. Trans Pa Acad Ophthalmol Otolaryngol. 1983 Fall;36(2):131–137. [PubMed] [Google Scholar]
- Kielar R. A. Toxocara canis endophthalmitis with low ELISA titer. Ann Ophthalmol. 1983 May;15(5):447–449. [PubMed] [Google Scholar]
- Klei T. R., McVay C. S., Dennis V. A., Coleman S. U., Enright F. M., Casey H. W. Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp Parasitol. 1990 Nov;71(4):393–405. doi: 10.1016/0014-4894(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Luxenberg M. N. An experimental approach to the study of intraocular Toxocara canis. Trans Am Ophthalmol Soc. 1979;77:542–602. [PMC free article] [PubMed] [Google Scholar]
- Maguire A. M., Green W. R., Michels R. G., Erozan Y. S. Recovery of intraocular Toxocara canis by pars plana vitrectomy. Ophthalmology. 1990 May;97(5):675–680. doi: 10.1016/s0161-6420(90)32528-9. [DOI] [PubMed] [Google Scholar]
- NICHOLS R. L. The etiology of visceral larva migrans. I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol. 1956 Aug;42(4 SECTION):349–362. [PubMed] [Google Scholar]
- OSHIMA T. Standardization of techniques for infecting mice with Toxocara canis and observations on the normal migration routes of the larvae. J Parasitol. 1961 Aug;47:652–656. [PubMed] [Google Scholar]
- Pollard Z. F. Long-term follow-up in patients with ocular toxocariasis as measured by ELISA titers. Ann Ophthalmol. 1987 May;19(5):167–169. [PubMed] [Google Scholar]
- Price J. A., Jr, Wadsworth J. A. An intraretinal worm. Report of a case of macular retinopathy caused by invasion of the retina by a worm. Arch Ophthalmol. 1970 Jun;83(6):768–770. doi: 10.1001/archopht.1970.00990030768018. [DOI] [PubMed] [Google Scholar]
- Rey A. NEMATODE ENDOPHTHALMITIS DUE TO TOXOCARA. Br J Ophthalmol. 1962 Oct;46(10):616–618. doi: 10.1136/bjo.46.10.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. NEMATODE ENDOPHTHALMITIS DUE TO TOXOCARA. Br J Ophthalmol. 1962 Oct;46(10):616–618. doi: 10.1136/bjo.46.10.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A. Early pars plana vitrectomy in chronic endophthalmitis of toxocariasis. Graefes Arch Clin Exp Ophthalmol. 1986;224(3):218–220. doi: 10.1007/BF02143057. [DOI] [PubMed] [Google Scholar]
- Searl S. S., Moazed K., Albert D. M., Marcus L. C. Ocular toxocariasis presenting as leukocoria in a patient with low ELISA titer to Toxocara canis. Ophthalmology. 1981 Dec;88(12):1302–1306. doi: 10.1016/s0161-6420(81)34860-x. [DOI] [PubMed] [Google Scholar]
- Siam A. L. Toxocaral chorio-retinitis. Treatment of early cases with photocoagulation. Br J Ophthalmol. 1973 Sep;57(9):700–703. doi: 10.1136/bjo.57.9.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorr E. M. Meandering ocular toxocariasis. Retina. 1984 Spring-Summer;4(2):90–96. doi: 10.1097/00006982-198400420-00003. [DOI] [PubMed] [Google Scholar]
- WILDER H. C. Nematode endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol. 1950 Nov-Dec;55:99–109. [PubMed] [Google Scholar]
- Watzke R. C., Oaks J. A., Folk J. C. Toxocara canis infection of the eye. Correlation of clinical observations with developing pathology in the primate model. Arch Ophthalmol. 1984 Feb;102(2):282–291. doi: 10.1001/archopht.1984.01040030226032. [DOI] [PubMed] [Google Scholar]
- Watzke R. C. Ocular Toxocara canis infection: clinical and experimental features. Trans New Orleans Acad Ophthalmol. 1983;31:263–271. [PubMed] [Google Scholar]
- Wilkinson C. P., Welch R. B. Intraocular toxocara. Am J Ophthalmol. 1971 Apr;71(4):921–930. doi: 10.1016/0002-9394(71)90267-4. [DOI] [PubMed] [Google Scholar]
- el Matri L., Ghorbal M., Ayadi A., Ben Naceur B., Triki M. F. Localisation oculaire à toxocara canis apparemment bilatérale. J Fr Ophtalmol. 1990;13(5):303–308. [PubMed] [Google Scholar]