Abstract
AIM—To study herpes simplex virus (HSV) DNA in tears from patients with atypical epithelial keratitis of unknown aetiology. METHODS—Tear samples were collected from 17 affected eyes of 17 consecutive patients suffering from epithelial keratitis in whom HSV keratitis was suspected but whose diagnosis was difficult on the basis of clinical manifestations alone. Using reduced sensitivity polymerase chain reaction (PCR), tear samples were tested for HSV DNA. Tears from the unaffected eyes of the 17 patients were also examined, along with 38 tear samples from 19 normal volunteers. Southern blot analysis was performed to confirm that amplified DNA bands were specific for HSV. Clinical correlation with photographs of corneal lesions was also investigated. RESULTS—HSV DNA was detected in tears from the affected eyes of eight of the 17 patients with suspected HSV keratitis. Tears from the affected eyes of the other patients were PCR negative, as were tears from the unaffected eyes of all 17 patients, and from the 38 normal eyes. There was no correlation between PCR results and clinical manifestation of keratitis. CONCLUSIONS—Based on the sensitivity of the PCR system, eight of 17 suspected HSV keratitis patients were confirmed as suffering from HSV keratitis. HSV keratitis should therefore be considered as a possible diagnosis in atypical epithelial keratitis.
Full Text
The Full Text of this article is available as a PDF (143.0 KB).
Figure 1 .
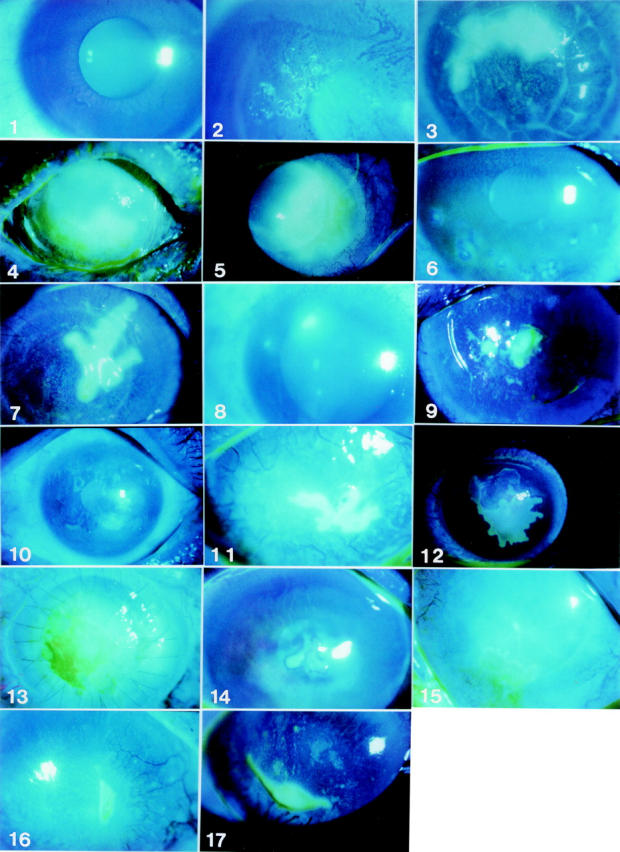
Fluorescein stained anterior segment photographs of 17 patients with atypical epithelial keratitis. Photograph numbers match patient numbers.
Figure 2 .
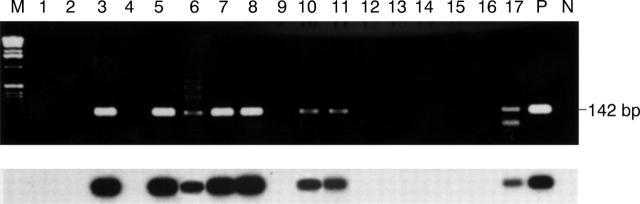
Detection of herpes simplex virus DNA in tear samples. (Top) Agarose gel electrophoresis of PCR products. Lanes 1-17 represent tears from 17 eyes of atypical epithelial keratitis patients. P=positive control; N=negative control. Patient Nos 3, 5, 6, 7, 8, 10, 11, 17 showed 142 bp amplified bands. (Bottom) Southern blot hybridisation confirms results of agarose gel electrophoresis. Lanes 1-P are same as those at the top.
Figure 3 .
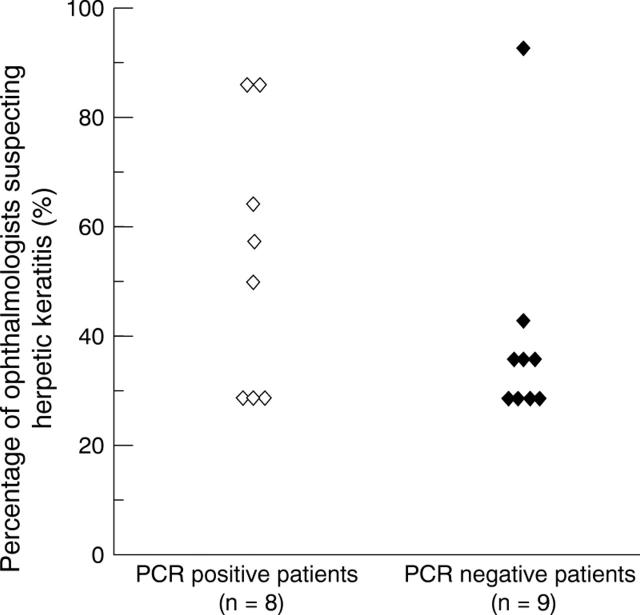
Distribution of percentages of 14 ophthalmologists suspecting herpetic keratitis in PCR positive and negative patients. Each lozenge matches one patient.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado J. A., Underwood J. L., Green W. R., Wu S., Murphy C. G., Hwang D. G., Moore T. E., O'Day D. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1994 Dec;112(12):1601–1609. doi: 10.1001/archopht.1994.01090240107034. [DOI] [PubMed] [Google Scholar]
- Beyer C. F., Arens M. Q., Hill J. M., Rose B. T., Hill G. A., Lin D. T. Penetrating keratoplasty in rabbits induces latent HSV-1 reactivation when corticosteroids are used. Curr Eye Res. 1989 Dec;8(12):1323–1329. doi: 10.3109/02713688909013913. [DOI] [PubMed] [Google Scholar]
- Boerman R. H., Arnoldus E. P., Raap A. K., Bloem B. R., Verhey M., van Gemert G., Peters A. C., van der Ploeg M. Polymerase chain reaction and viral culture techniques to detect HSV in small volumes of cerebrospinal fluid; an experimental mouse encephalitis study. J Virol Methods. 1989 Aug;25(2):189–197. doi: 10.1016/0166-0934(89)90032-3. [DOI] [PubMed] [Google Scholar]
- Cockerham G. C., Krafft A. E., McLean I. W. Herpes simplex virus in primary graft failure. Arch Ophthalmol. 1997 May;115(5):586–589. doi: 10.1001/archopht.1997.01100150588001. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Short G. A., Irvine A. R., Duker J. S., Margolis T. P. Acquired immunodeficiency syndrome--associated herpes simplex virus retinitis. Clinical description and use of a polymerase chain reaction--based assay as a diagnostic tool. Arch Ophthalmol. 1996 Jul;114(7):834–840. doi: 10.1001/archopht.1996.01100140048006. [DOI] [PubMed] [Google Scholar]
- Fox G. M., Crouse C. A., Chuang E. L., Pflugfelder S. C., Cleary T. J., Nelson S. J., Atherton S. S. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol. 1991 Feb;109(2):266–271. doi: 10.1001/archopht.1991.01080020112054. [DOI] [PubMed] [Google Scholar]
- Garweg J., Fenner T., Böhnke M., Schmitz H. An improved technique for the diagnosis of viral retinitis from samples of aqueous humor and vitreous. Graefes Arch Clin Exp Ophthalmol. 1993 Sep;231(9):508–513. doi: 10.1007/BF00921115. [DOI] [PubMed] [Google Scholar]
- Kumano Y., Manabe J., Hamamoto M., Kawano Y., Minagawa H., Fukumaki Y., Inomata H. Detection of varicella-zoster virus genome having a PstI site in the ocular sample from a patient with acute retinal necrosis. Ophthalmic Res. 1995;27(5):310–316. doi: 10.1159/000267740. [DOI] [PubMed] [Google Scholar]
- Mitchell S. M., Fox J. D., Tedder R. S., Gazzard B. G., Lightman S. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994 Aug;43(4):336–340. doi: 10.1002/jmv.1890430404. [DOI] [PubMed] [Google Scholar]
- Nishi M., Hanashiro R., Mori S., Masuda K., Mochizuki M., Hondo R. Polymerase chain reaction for the detection of the varicella-zoster genome in ocular samples from patients with acute retinal necrosis. Am J Ophthalmol. 1992 Nov 15;114(5):603–609. doi: 10.1016/s0002-9394(14)74491-5. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Yamamoto S., Nishida K., Okamoto S., Kinoshita S., Hayashi K., Manabe R. Demonstration of herpes simplex virus DNA in idiopathic corneal endotheliopathy. Am J Ophthalmol. 1991 Oct 15;112(4):419–423. doi: 10.1016/s0002-9394(14)76251-8. [DOI] [PubMed] [Google Scholar]
- Powell K. F., Anderson N. E., Frith R. W., Croxson M. C. Non-invasive diagnosis of herpes simplex encephalitis. Lancet. 1990 Feb 10;335(8685):357–358. doi: 10.1016/0140-6736(90)90648-o. [DOI] [PubMed] [Google Scholar]
- Rahhal F. M., Siegel L. M., Russak V., Wiley C. A., Tedder D. G., Weinberg A., Rickman L., Freeman W. R. Clinicopathologic correlations in acute retinal necrosis caused by herpes simplex virus type 2. Arch Ophthalmol. 1996 Nov;114(11):1416–1419. doi: 10.1001/archopht.1996.01100140616019. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Tei M., Nishida K., Kinoshita S. Polymerase chain reaction detection of herpes simplex virus in tear fluid from atypical herpetic epithelial keratitis after penetrating keratoplasty. Am J Ophthalmol. 1996 Nov;122(5):732–735. doi: 10.1016/s0002-9394(14)70497-0. [DOI] [PubMed] [Google Scholar]
- Wilhelmus K. R., Falcon M. G., Jones B. R. Bilateral herpetic keratitis. Br J Ophthalmol. 1981 Jun;65(6):385–387. doi: 10.1136/bjo.65.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Pavan-Langston D., Kinoshita S., Nishida K., Shimomura Y., Tano Y. Detecting herpesvirus DNA in uveitis using the polymerase chain reaction. Br J Ophthalmol. 1996 May;80(5):465–468. doi: 10.1136/bjo.80.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Pavan-Langston D., Tada R., Yamamoto R., Kinoshita S., Nishida K., Shimomura Y., Tano Y. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol. 1995 Jun;119(6):796–798. doi: 10.1016/s0002-9394(14)72788-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shimomura Y., Kinoshita S., Nishida K., Yamamoto R., Tano Y. Detection of herpes simplex virus DNA in human tear film by the polymerase chain reaction. Am J Ophthalmol. 1994 Feb 15;117(2):160–163. doi: 10.1016/s0002-9394(14)73071-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shimomura Y., Kinoshita S., Tano Y. Differentiating zosteriform herpes simplex from ophthalmic zoster. Arch Ophthalmol. 1994 Dec;112(12):1515–1516. doi: 10.1001/archopht.1994.01090240021012. [DOI] [PubMed] [Google Scholar]
- Yu D. D., Lemp M. A., Mathers W. D., Espy M., White T. Detection of varicella-zoster virus DNA in disciform keratitis using polymerase chain reaction. Arch Ophthalmol. 1993 Feb;111(2):167–168. doi: 10.1001/archopht.1993.01090020021010. [DOI] [PubMed] [Google Scholar]
- de Boer J. H., Verhagen C., Bruinenberg M., Rothova A., de Jong P. T., Baarsma G. S., Van der Lelij A., Ooyman F. M., Bollemeijer J. G., Derhaag P. J. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996 Jun;121(6):650–658. doi: 10.1016/s0002-9394(14)70631-2. [DOI] [PubMed] [Google Scholar]