Abstract
BACKGROUND/AIMS—To investigate the in vitro effect of a short time exposure to the anthracycline idarubicin on proliferation, protein synthesis, and motility of human Tenon's capsule fibroblasts in comparison with the antitumour antibiotic mitomycin C. METHODS—After determination of effective concentrations of idarubicin, fibroblasts of the human Tenon's capsule were exposed to idarubicin or mitomycin C at concentrations ranging from 0.1 µg/ml to 1 µg/ml or from 2.5 µg/ml to 250 µg/ml, respectively, for 0.5, 2, or 5 minutes and cultured for 60 days. Cell death by apoptosis caused by idarubicin treatment was confirmed by Hoechst 33258 staining. Further proliferation was explored by cell counting and by 3H-thymidine uptake. Protein synthesis was measured by 3H-proline uptake and motility was assessed by agarose droplet motility assay. RESULTS—Idarubicin is able to exert toxicity and to induce apoptosis during a short time exposure of 0.5 minutes at concentrations of 0.3-1 µg/ml resulting in a significant reduction in cell number compared with the control after 60 days. For mitomycin C, higher concentrations and longer expositions were necessary. Even after treatment with 1 µg/ml idarubicin or 250 µg/ml mitomycin C a few cells were able to incorporate 3H-thymidine. 3H-proline uptake up to 10 days after exposure to 0.3 µg/ml idarubicin was found not to be decreased. Cell motility was reduced after treatment with 1 µg/ml idarubicin for 5 minutes or with 250 µg/ml mitomycin C for 2 or 5 minutes. For low mitomycin C concentrations, an increase in motility was found during the first 10 days. CONCLUSION—Idarubicin reduces proliferation of human Tenons's capsule fibroblasts after incubation for 0.5 minutes at concentrations as low as 0.3-1 µg/ml. In comparison, mitomycin C requires longer exposure times and higher doses for equal results. Therefore, idarubicin may be useful in the prevention of glaucoma filtering surgery failure.
Full Text
The Full Text of this article is available as a PDF (116.4 KB).
Figure 1 .
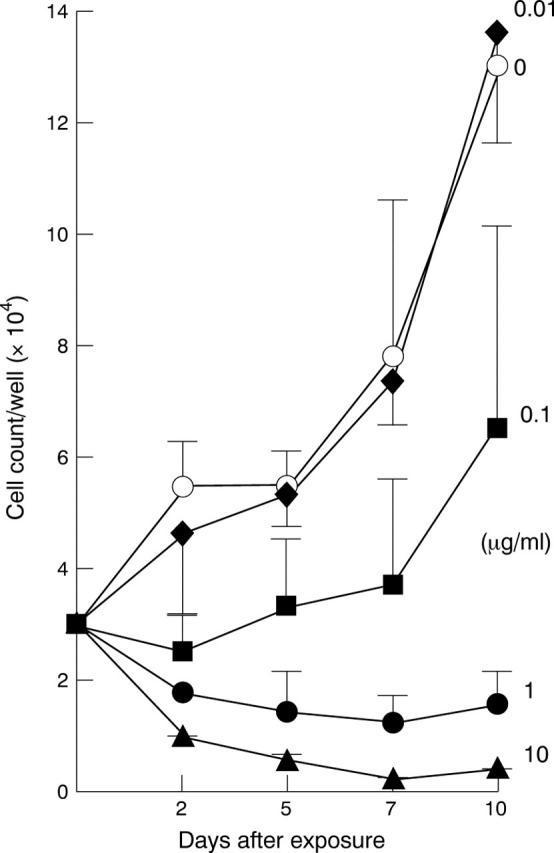
Effect of the idarubicin concentration on proliferation of fibroblasts of the human Tenon's capsule. To determine effective concentrations, cells of two different patients were treated in triplicate with different concentrations ranging from 0.01 µg/ml to 10 µg/ml for 5 minutes. Afterwards fibroblasts were cultured for 10 days and counted (six samples per point) at day 2, 5, 7, and 10 after exposure to idarubicin. Additional concentrations are shown in Table 1.
Figure 2 .
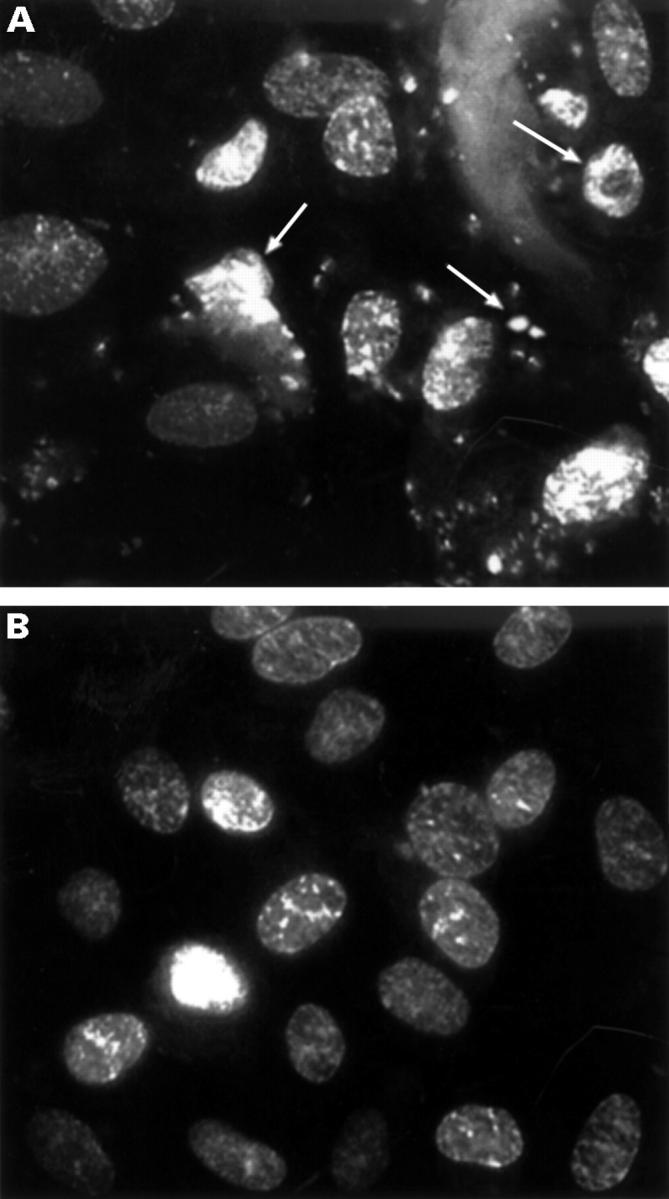
Assessment of apoptosis. To confirm cell death by apoptosis in the initial phase of the experiment Tenon's capsule fibroblasts were exposed to 1 µg/ml idarubicin for 0.5 minutes (A) or to buffer (B) and stained with Hoechst 33258. The cells treated with the cytostatic agent show the typical morphological feature of apoptosis with chromatine condensation, DNA fragmentation, and release of apoptotic bodies (arrows).
Figure 3 .
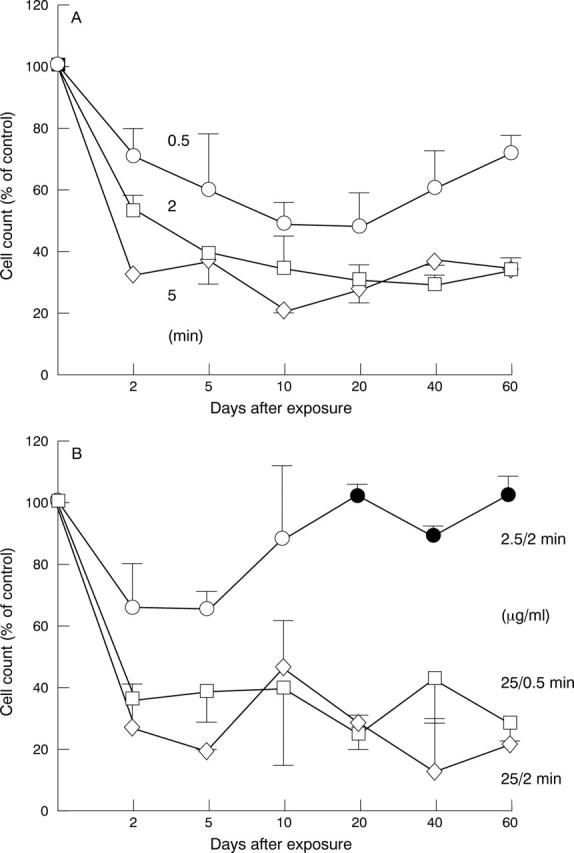
Comparison of the effect of idarubicin and mitomycin C treatment on the proliferation of fibroblasts during long term culturing. (A) Cells were exposed to 0.3 µg/ml idarubicin for 0.5, 2, and 5 minutes. (B) Cells were exposed to mitomycin C at a concentration of 2.5 µg/ml mitomycin C for 2 minutes or 25 µg/ml for 0.5 and 2 minutes. The data shown reflect the mean value of fibroblast preparations of two different patients (16 samples per point). Filled symbols, cell number not significantly reduced compared with the control (p >0.05).
Figure 4 .
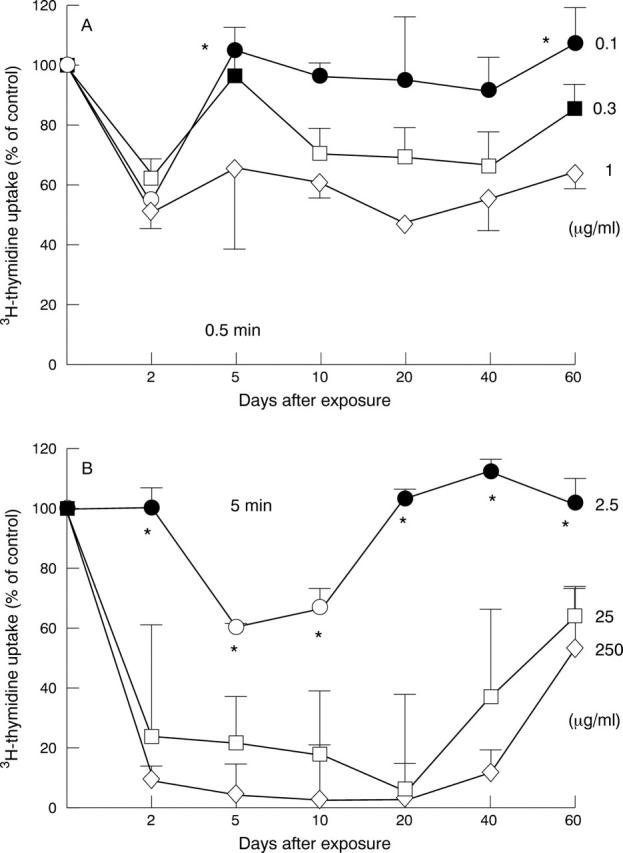
Effect of idarubicin and mitomycin C on DNA replication. The incorporation of 3H-thymidine was measured for fibroblasts treated with idarubicin at concentrations of 0.1-1 µg/ml for 0.5 minutes (A) or with mitomycin C at concentrations of 2.5-250 µg/ml for 5 minutes (B) during long term culture. The experiment was done in triplicate for fibroblast preparations of two different patients. Filled symbols, 3H-thymidine uptake not significantly reduced compared with the control (p>0.05). Asterisks, cell number not significantly reduced compared with the control (p>0.05).
Figure 5 .
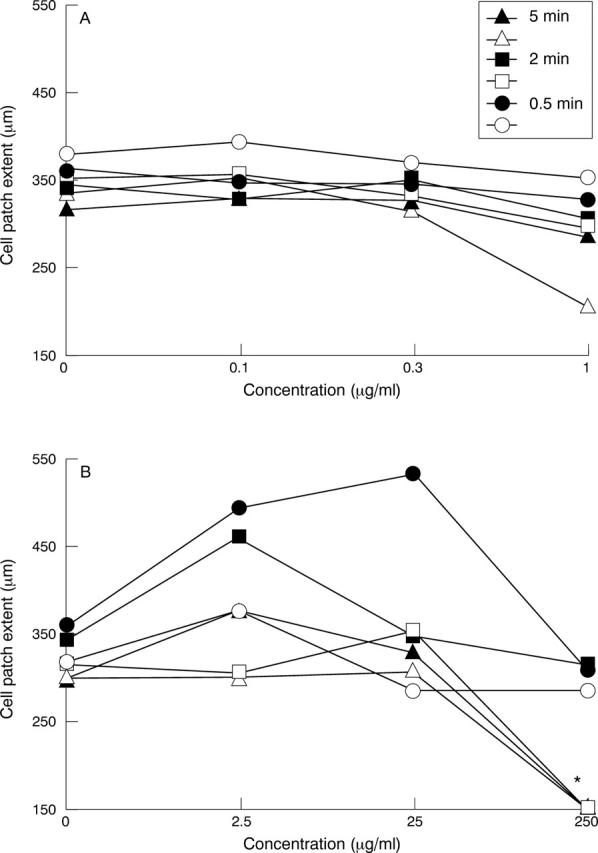
Effect of idarubicin and mitomycin C on cell motility. Fibroblasts were exposed to idarubicin at concentrations of 0.1-1 µg/ml for 0.5-5 minutes (A) or to mitomycin C at concentrations of 2.5-250 µg/ml for 0.5-5 minutes (B). Motility was assessed by agarase droplet motility assay 10 and 60 days after exposure twice for fibroblast preparations of two different patients. Standard deviations ranged from 2.65 µm to 117.2 µm, mean 44.8 µm for the idarubicin experiments and from 0 µm to 143.2 µm, mean 61.5 µm for the mitomycin C experiments. Filled symbols, 10 days after exposure; open symbols, 60 days after exposure. Asterisk, no cell patch.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman E., McBride M. Comparative cellular pharmacology of daunorubicin and idarubicin in human multidrug-resistant leukemia cells. Blood. 1992 Jun 15;79(12):3267–3273. [PubMed] [Google Scholar]
- Crowston J. G., Akbar A. N., Constable P. H., Occleston N. L., Daniels J. T., Khaw P. T. Antimetabolite-induced apoptosis in Tenon's capsule fibroblasts. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):449–454. [PubMed] [Google Scholar]
- Gieseler F., Biersack H., Brieden T., Manderscheid J., Nüssler V. Cytotoxicity of anthracyclines: correlation with cellular uptake, intracellular distribution and DNA binding. Ann Hematol. 1994;69 (Suppl 1):S13–S17. doi: 10.1007/BF01757349. [DOI] [PubMed] [Google Scholar]
- Jampel H. D., McGuigan L. J., Dunkelberger G. R., L'Hernault N. L., Quigley H. A. Cellular proliferation after experimental glaucoma filtration surgery. Arch Ophthalmol. 1988 Jan;106(1):89–94. doi: 10.1001/archopht.1988.01060130095036. [DOI] [PubMed] [Google Scholar]
- Jampel H. D., Pasquale L. R., Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin C. Arch Ophthalmol. 1992 Aug;110(8):1049–1050. doi: 10.1001/archopht.1992.01080200029011. [DOI] [PubMed] [Google Scholar]
- Khaw P. T., Sherwood M. B., MacKay S. L., Rossi M. J., Schultz G. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon's capsule fibroblasts. Arch Ophthalmol. 1992 Aug;110(8):1150–1154. doi: 10.1001/archopht.1992.01080200130040. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y., Kawase K., Matsushita H., Minobe M. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch Ophthalmol. 1991 Dec;109(12):1693–1698. doi: 10.1001/archopht.1991.01080120077030. [DOI] [PubMed] [Google Scholar]
- Lavin M. J., Wormald R. P., Migdal C. S., Hitchings R. A. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990 Nov;108(11):1543–1548. doi: 10.1001/archopht.1990.01070130045027. [DOI] [PubMed] [Google Scholar]
- Madhavan H. N., Rao S. B., Vijaya L., Neelakantan A. In vitro sensitivity of human Tenon's capsule fibroblasts to mitomycin C and its correlation with outcome of glaucoma filtration surgery. Ophthalmic Surg. 1995 Jan-Feb;26(1):61–67. [PubMed] [Google Scholar]
- McGuigan L. J., Quigley H. A., Lutty G., Enger C., Young E. The effects of D-penicillamine and daunorubicin on conjunctival fibroblast proliferation and collagen synthesis. Invest Ophthalmol Vis Sci. 1988 Jan;29(1):112–118. [PubMed] [Google Scholar]
- Occleston N. L., Daniels J. T., Tarnuzzer R. W., Sethi K. K., Alexander R. A., Bhattacharya S. S., Schultz G. S., Khaw P. T. Single exposures to antiproliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci. 1997 Sep;38(10):1998–2007. [PubMed] [Google Scholar]
- Richter C. U., Shingleton B. J., Bellows A. R., Hutchinson B. T., O'Connor T., Brill I. The development of encapsulated filtering blebs. Ophthalmology. 1988 Sep;95(9):1163–1168. doi: 10.1016/s0161-6420(88)33041-1. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Weiss H. S. Bleb leak with hypotony after laser suture lysis and trabeculectomy with mitomycin C. Arch Ophthalmol. 1992 Aug;110(8):1049–1049. doi: 10.1001/archopht.1992.01080200029010. [DOI] [PubMed] [Google Scholar]
- Scott D. R., Quigley H. A. Medical management of a high bleb phase after trabeculectomies. Ophthalmology. 1988 Sep;95(9):1169–1173. doi: 10.1016/s0161-6420(88)33031-9. [DOI] [PubMed] [Google Scholar]
- Sherwood M. B., Grierson I., Millar L., Hitchings R. A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology. 1989 Mar;96(3):327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- Sibayan S. A., Latina M. A., Sherwood M. E., Flotte T. J., White K. Apoptosis and morphologic changes in drug-treated trabecular meshwork cells in vitro. Exp Eye Res. 1998 May;66(5):521–529. doi: 10.1006/exer.1997.0458. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Rackstraw C., Cotter F. DNA fragmentation as a consequence of cell cycle traverse in doxorubicin- and idarubicin-treated human lymphoma cells. Ann Hematol. 1994;69 (Suppl 1):S7–11. doi: 10.1007/BF01757348. [DOI] [PubMed] [Google Scholar]
- Tanihara H., Yokoi N., Komuro A., Honda Y., Kinoshita S. Prolonged impairment of peripheral corneal epithelium barrier function after successful trabeculectomy. Am J Ophthalmol. 1997 Apr;123(4):487–493. doi: 10.1016/s0002-9394(14)70174-6. [DOI] [PubMed] [Google Scholar]
- Testi R., Mattii L., Di Simone D., Zaccaro L., Malvaldi G., Grassi B., Petrini M. Evaluation of resistance index of several anticancer agents on parental and resistant P-388 cell lines. Leuk Res. 1995 Apr;19(4):257–261. doi: 10.1016/0145-2126(94)00157-6. [DOI] [PubMed] [Google Scholar]
- Yaldo M. K., Stamper R. L. Long-term effects of mitomycin on filtering blebs. Lack of fibrovascular proliferative response following severe inflammation. Arch Ophthalmol. 1993 Jun;111(6):824–826. doi: 10.1001/archopht.1993.01090060112033. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Varani J., Soong H. K., Lichter P. R. Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology. 1990 Sep;97(9):1204–1210. doi: 10.1016/s0161-6420(90)32456-9. [DOI] [PubMed] [Google Scholar]


