Abstract
BACKGROUND/AIMS—Povidone-iodine (PVP -I) is applied for microbial decontamination of human eyes donated for transplantation. Concentrations and immersion times vary greatly. The effectiveness and toxicity of PVP-I were assessed for different decontamination protocols. METHODS—Human donor eyes and corneas were immersed in different concentrations (5-100 mg/ml) of PVP-I for different times (2-30 minutes). The penetration of iodine into the corneal tissue was assessed by x ray microanalysis. Microbial contamination was determined by taking cultures of the limbal areas and storage solutions and by incubation of the corneoscleral buttons in antibiotic-free culture medium. Cytotoxicity of PVP-I for corneal fibroblasts in culture was assessed using the MTT assay. RESULTS—Depending on concentration and immersion time iodine was found to penetrate into the epithelium, Bowman's layer, and stroma in amounts equivalent to 2-40 mg/ml PVP-I. The MTT assay demonstrated that 2.5 mg/ml PVP-I caused total damage to fibroblasts in vitro. Rinsing eyes with tap water and subsequent immersion in PVP-I reduced the rate of contamination from 82 out of 106 to 69 out of 106 and 37 out of 106, respectively. Antibiotics in the storage medium further reduced contamination from about 40% to 3%. Microbial contamination was not reduced by increasing the concentration and immersion times beyond 5 mg/ml PVP-I for 2 minutes. CONCLUSION—Immersion of human donor eyes in 5 mg/ml PVP-I solution for 2 minutes significantly reduces microbial contamination of donor corneas without relevant penetration of iodine into the corneal layers. Higher PVP-I concentrations and longer immersion times do not further reduce contamination, whereas the amount of iodine penetrating the corneal layers is elevated above the level cytotoxic for corneal fibroblasts. In view of this, concentrations above 5 mg/ml of PVP-I and immersion periods over 2 minutes are not recommended for reduction of the contamination rate of donor eyes.
Full Text
The Full Text of this article is available as a PDF (177.0 KB).
Figure 1 .
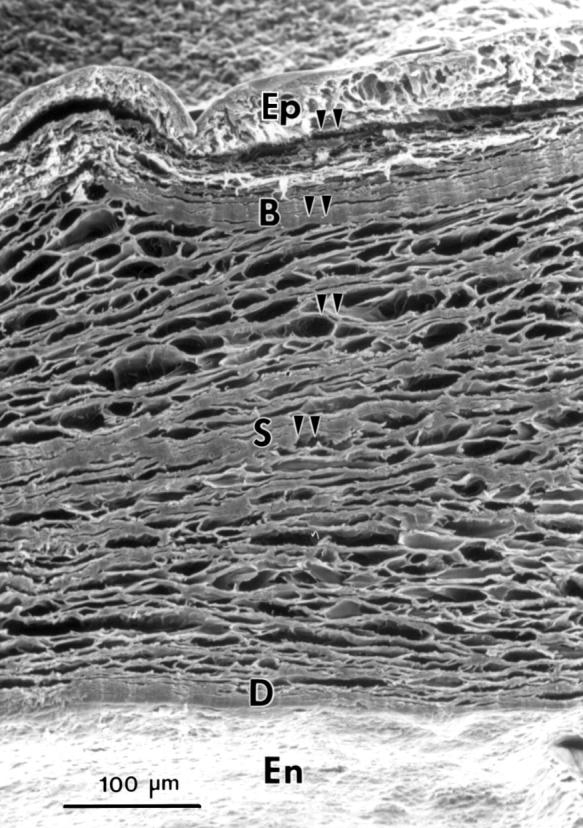
Scanning electron micrograph of the transverse side of a corneal piece. Epithelium (Ep), Bowman's layer (B), stroma (S), Descemet's membrane (D), and endothelium (En). Arrowheads indicate the sites of x ray microanalysis.
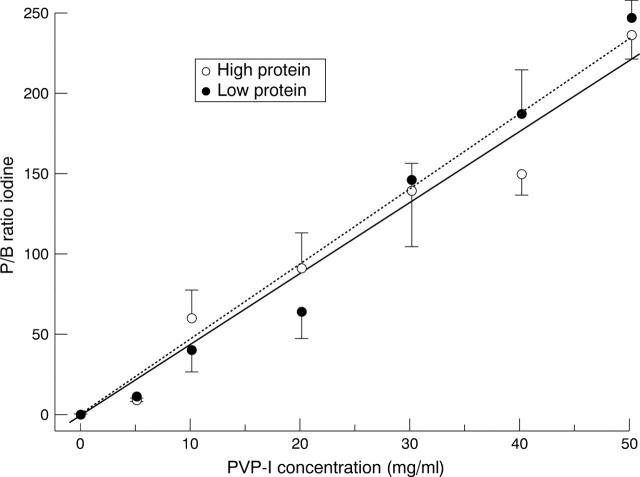
Figure 2 .
PVP-I concentration and P/B ratio of iodine for standard samples (see Methods) showing the significant correlations for high protein (R=0.98) and low protein (R=0.99) standards. P/B ratios for iodine expressed as mean (SEM) of values assessed in three experiments.
Figure 3 .
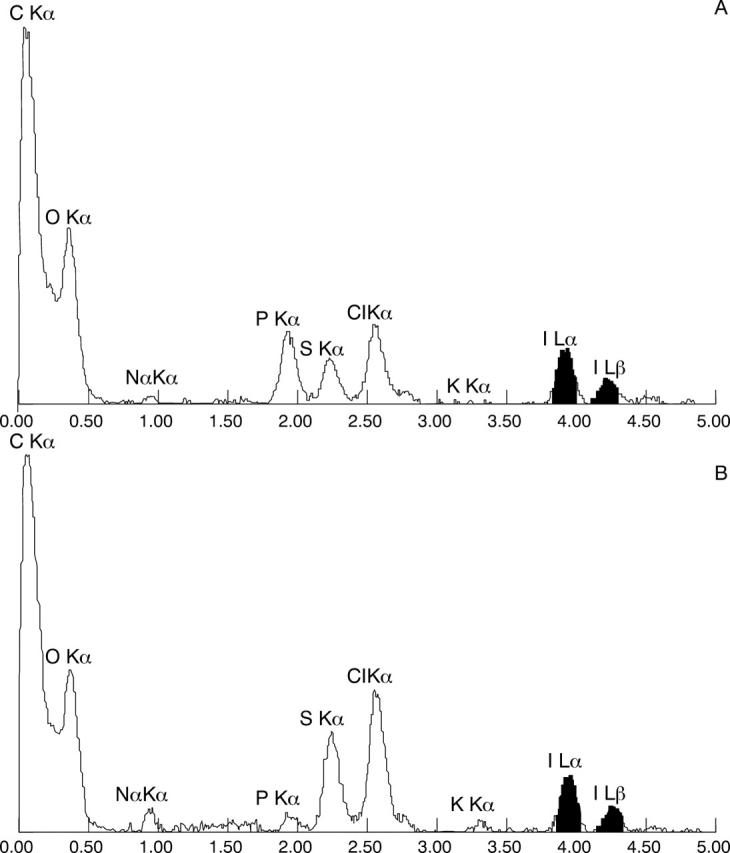
EDAX spectra of a cornea immersed in 50 mg/ml PVP-I for 10 minutes analysed in the basal layer of the epithelium (A) and anterior stroma (B). For details see text.
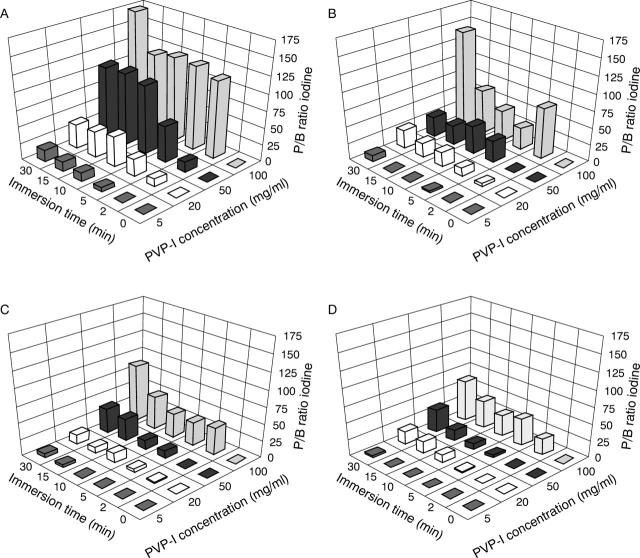
Figure 4 .
Penetration of iodine in the epithelium (A), Bowman's layer (B), anterior stroma (C), and mid-stroma (D) of the cornea expressed as P/B ratio for iodine after immersion in 5, 20, 50, 100 mg/ml PVP-I solution for different time periods up to 30 minutes. Results are representative of five experiments.
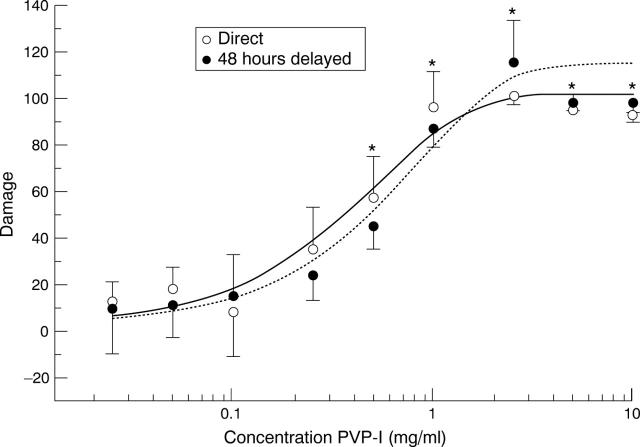
Figure 5 .
Dose/response curve of PVP-I solutions for human corneal fibroblasts. Damage was assessed with the MTT assay directly after exposure of the cells to the PVP-I and 48 hours later. Mean (SD) of the means obtained in five experiments in eightfold. Asterisks indicate damage significantly different from controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage W. J., Easty D. L. Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):16–24. [PubMed] [Google Scholar]
- Badenoch P. R., Alfrich S. J., Wedding T. R., Coster D. J. Effectiveness of a decontamination method for donor corneas. Br J Ophthalmol. 1988 Mar;72(3):225–227. doi: 10.1136/bjo.72.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelman R. L., Holland B. W., Anderson R. L. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982 Apr;15(4):635–639. doi: 10.1128/jcm.15.4.635-639.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg-Ans J., Badsberg E., Rasmussen J. FREQUENCY OF INFECTION IN DONOR EYES POST MORTEM: A METHOD OF OBTAINING STERILE EYES FOR CORNEAL GRAFTING. Br J Ophthalmol. 1962 Jun;46(6):365–368. doi: 10.1136/bjo.46.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCTOR D., HUGHES I. Prophylactic use of neosporin for donor eyes. Am J Ophthalmol. 1958 Sep;46(3 Pt 1):351–353. doi: 10.1016/0002-9394(58)90260-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Rosman I. Sex chromosome loss and clones characterized by autosomal abnormality in cultured corneal fibroblasts of man. Cytogenet Cell Genet. 1984;38(1):29–33. doi: 10.1159/000132025. [DOI] [PubMed] [Google Scholar]
- Garcia-Ferrer F. J., Murray P. R., Pepose J. S. Corneal endothelial toxicity of DexSol corneal storage medium supplemented with povidone-iodine. Arch Ophthalmol. 1992 Nov;110(11):1519–1520. doi: 10.1001/archopht.1992.01080230017007. [DOI] [PubMed] [Google Scholar]
- Garweg J., Böhnke M., Sabow D., Draeger J. Bakterielle Kontamination bei der Hornhautkonservierung: Keime und deren Herkunft. Fortschr Ophthalmol. 1989;86(4):287–290. [PubMed] [Google Scholar]
- Goldman K. N., Centifanto Y., Kaufman H. E., Slappey T. E. Prevention of surface bacterial contamination of donor corneas. Arch Ophthalmol. 1978 Dec;96(12):2277–2280. doi: 10.1001/archopht.1978.03910060573023. [DOI] [PubMed] [Google Scholar]
- Gopinathan U., Reddy M. K., Nadkarni M. S., Dasari S., Rao G. N. Antimicrobial effect of ciprofloxacin, povidone-iodine, and gentamicin in the decontamination of human donor globes. Cornea. 1998 Jan;17(1):57–61. doi: 10.1097/00003226-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Gross A., Cofone L., Huff M. B. Iodine inactivating agent in surgical scrub testing. Arch Surg. 1973 Feb;106(2):175–178. doi: 10.1001/archsurg.1973.01350140037012. [DOI] [PubMed] [Google Scholar]
- Hagenah M., Böhnke M., Engelmann K., Winter R. Incidence of bacterial and fungal contamination of donor corneas preserved by organ culture. Cornea. 1995 Jul;14(4):423–426. doi: 10.1097/00003226-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Hall T. A. Biological X-ray microanalysis. J Microsc. 1979 Sep;117(1):145–163. doi: 10.1111/j.1365-2818.1979.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Hoppenreijs V. P., Pels E., Vrensen G. F., Felten P. C., Treffers W. F. Platelet-derived growth factor: receptor expression in corneas and effects on corneal cells. Invest Ophthalmol Vis Sci. 1993 Mar;34(3):637–649. [PubMed] [Google Scholar]
- Keates R. H., Mishler K. E., Riedinger D. Bacterial contamination of donor eyes. Am J Ophthalmol. 1977 Nov;84(5):617–619. doi: 10.1016/0002-9394(77)90374-9. [DOI] [PubMed] [Google Scholar]
- Mac Rae S. M., Brown B., Edelhauser H. F. The corneal toxicity of presurgical skin antiseptics. Am J Ophthalmol. 1984 Feb;97(2):221–232. doi: 10.1016/s0002-9394(14)76094-5. [DOI] [PubMed] [Google Scholar]
- Mindrup E. A., Dubbel P. A., Doughman D. J. Betadine decontamination of donor globes. Cornea. 1993 Jul;12(4):324–329. doi: 10.1097/00003226-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nash R. W., Lindquist T. D., Kalina R. E. An evaluation of saline irrigation and comparison of povidone-iodine and antibiotic in the surface decontamination of donor eyes. Arch Ophthalmol. 1991 Jun;109(6):869–872. doi: 10.1001/archopht.1991.01080060133041. [DOI] [PubMed] [Google Scholar]
- Pardos G. J., Gallagher M. A. Microbial contamination of donor eyes. A retrospective study. Arch Ophthalmol. 1982 Oct;100(10):1611–1613. doi: 10.1001/archopht.1982.01030040589006. [DOI] [PubMed] [Google Scholar]
- Pels E., Nuyts R. M., Breebaart A. C., Hartmann C. Rapid quantitative assays for corneal endothelial cell viability in vitro. Cornea. 1993 Jul;12(4):289–294. doi: 10.1097/00003226-199307000-00003. [DOI] [PubMed] [Google Scholar]
- Polack F. M., Locatcher-Khorazo D., Gutierrez E. Bacteriologic study of "donor" eyes. Evaluation of antibacterial treatments prior to corneal grafting. Arch Ophthalmol. 1967 Aug;78(2):219–225. doi: 10.1001/archopht.1967.00980030221018. [DOI] [PubMed] [Google Scholar]
- ROLLINS H. J., Jr, STOCKER F. W. BACTERIAL FLORA AND PREOPERATIVE TREATMENT OF DONOR CORNEAS. Am J Ophthalmol. 1965 Feb;59:247–249. [PubMed] [Google Scholar]
- RYCROFT P. METHOD FOR THE PRESERVATION AND STERILIZATION OF FRESH DONOR MATERIAL FOR FULL-THICKNESS KERATOPLASTY BY GRAMYCETIN. Br J Ophthalmol. 1965 May;49:251–258. doi: 10.1136/bjo.49.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter E., Götze J., Trute K., Strache S., Schmidt G., Gliem H. Beitrag zum Umfang bakterieller Kontaminationen bei Keratoplastik-Spenderaugen post mortem. Klin Monbl Augenheilkd. 1990 Feb;196(2):70–75. doi: 10.1055/s-2008-1046133. [DOI] [PubMed] [Google Scholar]
- Roomans G. M. Problems in quantitative x-ray microanalysis of biological specimens. Scan Electron Microsc. 1980;(Pt 2):309–320. [PubMed] [Google Scholar]
- Roomans G. M. Quantitative X-ray microanalysis of biological specimens. J Electron Microsc Tech. 1988 May;9(1):19–43. doi: 10.1002/jemt.1060090104. [DOI] [PubMed] [Google Scholar]
- Sobottka Ventura A. C., Engelmann K., Dahinden C., Böhnke M. Endotoxins modulate the autocrine function of organ cultured donor corneas and increase the incidence of endothelial cell death. Br J Ophthalmol. 1997 Dec;81(12):1093–1098. doi: 10.1136/bjo.81.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling S., Sørensen I. G. Decontamination of cadaver corneas. Acta Ophthalmol (Copenh) 1981 Feb;59(1):126–133. doi: 10.1111/j.1755-3768.1981.tb06720.x. [DOI] [PubMed] [Google Scholar]
- White J. H. Fungal contamination of donor eyes. Br J Ophthalmol. 1969 Jan;53(1):30–33. doi: 10.1136/bjo.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H. Assessment of possible toxic effects of polyvinylpyrrolidone-iodine upon the human eye in conjunction with cataract extraction. An endothelial specular microscope study. Acta Ophthalmol (Copenh) 1982 Dec;60(6):955–960. doi: 10.1111/j.1755-3768.1982.tb00627.x. [DOI] [PubMed] [Google Scholar]
- York K. K., Miller S., Gaster R. N., Burstein N. L. Polyvinylpyrrolidone iodine: corneal toxicology and epithelial healing in a rabbit model. J Ocul Pharmacol. 1988 Winter;4(4):351–358. doi: 10.1089/jop.1988.4.351. [DOI] [PubMed] [Google Scholar]
- Zamora J. L. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986 Mar;151(3):400–406. doi: 10.1016/0002-9610(86)90477-0. [DOI] [PubMed] [Google Scholar]