Abstract
Using an in vitro culture system, we observed the migration of malaria ookinetes on the surface of the mosquito midgut and invasion of the midgut epithelium. Ookinetes display constrictions during migration to the midgut surface and a gliding motion once on the luminal midgut surface. Invasion of a midgut cell always occurs at its lateral apical surface. Invasion is rapid and is often followed by invasion of a neighboring midgut cell by the ookinete. The morphology of the invaded cells changes dramatically after invasion, and invaded cells die rapidly. Midgut cell death is accompanied by activation of a caspase-3-like protease, suggesting cell death is apoptotic. The events occurring during invasion were identical for two different species of Plasmodium and two different genera of mosquitoes; they probably represent a universal mechanism of mosquito midgut penetration by the malaria parasite.
The transmission of malaria to a new vertebrate host depends on the successful passage of the malaria ookinete through the mosquito midgut, transformation to an oocyst on the external midgut wall, and the multiplication and migration of sporozoites into the lumen of the insect's salivary gland. After ingestion by its vector mosquito in a blood meal taken from an infected vertebrate host, male and female gametes form a zygote, which transforms into an ookinete in the lumen of the midgut. Before it can develop into an oocyst, however, the ookinete must escape from the blood meal and traverse the midgut epithelium to reach the midgut's basement membrane (1). Although several studies by light and transmission electron microscopy (TEM) have been directed at the ookinete's invasion of midgut cells (2–8), none have succeeded in producing a mechanistic description of the invasion process.
The development in the past of methods to culture various stages of Plasmodium or to observe their behavior in vitro has been of great importance in our ability to study the malaria parasite (9–15). With this history in mind, we developed an in vitro system to observe ookinete migration on the mosquito midgut surface and penetration of midgut cells. We here report the mechanism and kinetics of midgut cell invasion by the malaria ookinete.
Materials and Methods
Parasite and Mosquito Cultivation.
The 8A strain of the avian malaria parasite Plasmodium gallinaceum and the Liverpool/black eye strain of Aedes aegypti were used throughout this study. Parasites were maintained in white Leghorn chickens by serial blood passages. In addition, a standard laboratory strain of Anopheles stephensi was used, as well as the ANKA strain of Plasmodium berghei, which was maintained in BALB/c mice by serial blood passages. Mosquitoes were raised and fed by using standard techniques (16, 17).
Invasion Assay.
The midgut was removed from bloodfed A. aegypti mosquitoes and was cut longitudinally into two halves (18). The midgut epithelia were cleaned of the blood meal and gently spread out on a glass slide in M199 medium supplemented with 2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin (ookinete medium) with the luminal surface facing up.
Zygotes from P. gallinaceum were purified as described previously (13, 19) and were allowed to develop into ookinetes in ookinete medium. A published method was used for cultivation and purification of ookinetes from P. berghei (20). However, instead of using centrifugation to force the ookinetes in contact with the midguts (18, 21), a droplet of the ookinete suspension was layered over the midguts and incubated briefly to allow ookinete binding to the midgut surface by gravity.
The parasite-coated midguts were transferred to another glass slide that had two 80-μm-thick strips of self-adherent Teflon tape (TEMP-R-TAPE, The Connecticut Hard Rubber Co., New Haven, CT) attached perpendicularly to the long axis of the slide, leaving an open area approximately 1 cm wide between them. The midguts were deposited between the strips and gently spread out with dissection needles, leaving the luminal midgut surface facing upward. A cover glass was placed on top, forming a bridge between the two strips of Teflon tape and trapping the flattened midguts in the area created by the Teflon spacers. The resulting chamber was filled with ookinete medium supplemented with 10% heat-inactivated chicken serum (adhesion medium) and sealed on the sides with silicon grease to prevent evaporation. This culture system permitted high-resolution differential interference contrast (DIC), polarized light, and wide-field fluorescence video microscopy studies of the complete invasion process. The midguts remained alive in the chamber for approximately 2–3 h, as determined by the morphology of the midgut cells.
Ethidium homodimer (10 μM), propidium iodide (2 μg/ml), and YO-PRO-1 (200 nM) were obtained from Molecular Probes and were diluted in adhesion medium. The dyes were added to the medium in the micro chamber and were continuously present during the live observations.
PhiPhiLux-G1D2 (OncoImmunin, Gaithersburg, MD) was used to detect caspase activation in live midgut cells (22). A 10-μM solution of PhiPhiLux-G1D2 in adhesion medium was added to freshly dissected midguts and incubated for 10 min to preload the midgut cells with the dye. Excess dye was washed away, and the midguts were incubated with ookinetes and placed in a micro chamber as described above.
Microscopy.
A Zeiss Axiophot microscope equipped with a ×100 Plan Apochromat objective, a ×100 strain-free objective, DIC prisms, a polarizer and analyzer, a Dage/MTI Series 68 video camera (Dage-MTI, Michigan City, IN), and a Hamamatsu Model C2400–68 low-light-level video camera (Hamamatsu, Bridgewater, NJ) were used. All microscopy procedures were performed by using routine video and microscopy methods. Time code was added to the video data, and images were collected in image-pro, ver. 4.0 (Media Cybernetics, Silver Spring, MD) or with a Sony U-matic Professional video tape recorder. This setup allowed us to observe and record high-resolution images of the midgut surface and the midgut cells throughout the thickness of the midgut epithelium.
Data Analysis.
The rate of ookinete movement was quantified from 96 min of constricted movement and 92 min of gliding movement on videotape (see Table 1). Rates were calculated by serially isolating individual frames from a video sequence at 5- to 15-sec intervals, measuring the distance traveled by the parasite, and calculating the time interval. Distances and times for all time intervals corresponding to a specific type of movement were added to compute the mean rates of movement.
Table 1.
Quantification of Plasmodium gallinaceum ookinete migration and invasion of Aedes aegypti midguts
| Event | Mean ± standard deviation | Range (min–max) | Time or number of events |
|---|---|---|---|
| Movement through MN, μm/min* | 1.9 | 0–10.8 | 96.0 min |
| Movement on cell surface, μm/min† | 15.1 | 0–85.2 | 91.8 min |
| Time for complete invasion, min‡ | 1.3 ± 1.8 | 0.35–8.3 | 17 events¶ |
| Time for refractive index change, min§ | 1.0 ± 1.4 | 0–4.6 | 18 events¶ |
With one or more constrictions.
Without constrictions.
Elapsed time between onset of penetration and complete interiorization.
Elapsed time between onset of penetration and refractive index change of midgut cell.
The number of events used for quantification is lower than total number observed because some parameters were not recorded continuously in a small number of events.
The time for the invasion process was averaged over 19 invasion events. Times for the change of refractive index of invaded cells were measured and averaged in the same manner.
Results
Ookinete-coated mosquito midgut epithelia were placed in a micro chamber on a glass slide, permitting observations of ookinete movement on the midgut surface and invasion of the epithelial cells. Initially, ookinetes do not come into direct contact with the microvillar surface of midgut epithelial cells. They become enmeshed in an extensive microvilli-associated network (MN) consisting of fine membranous strands approximately 20–50 nm in diameter (21, 23) located between the luminal midgut contents and the microvilli. The MN can be visualized in fixed midguts by scanning and TEM and in live midguts by interferometry (unpublished results). Movement of the ookinetes through the MN is slow (Table 1) and is characterized by the formation of constrictions along the length of the ookinete (21). Once the ookinete is in direct contact with the midgut surface, it moves more rapidly (Table 1) by gliding motility, without the formation of constrictions. The parasite migrates along the crevices between midgut epithelial cells and moves in a discontinuous fashion, starting and stopping frequently. Invasion of midgut cells often occurred after extensive gliding (up to 30 min) of the ookinete over the midgut surface.
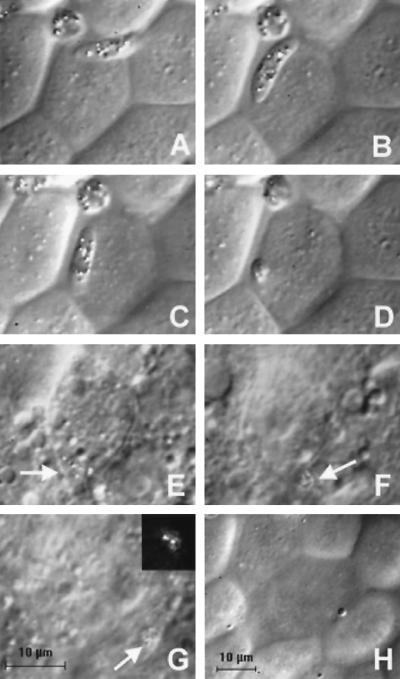
We observed 19 individual invasion events involving P. gallinaceum ookinetes and A. aegypti midgut cells. These events have many common and constant features. In the course of its movement in the crevices between midgut cells (Fig. 1 A and B), the anterior end of the ookinete is located immediately adjacent to the region containing midgut epithelial junctional complexes (Fig. 1 A and B). From its position between the apical free margins of two midgut cells, the ookinete invades the lateral apical surface (the region of the apical cell surface that is adjacent to the cell junctions) of one of the midgut cells (Fig. 1 C and D). This process is most clearly visualized when the midgut is well flattened under the cover glass, with minimal topological variation between apical cell surfaces and the regions of cell–cell contact (Fig. 1). When the crevices between midgut cells are more prominent, the ookinete can be seen moving deeply into the crevice between the apical margins of two midgut cells (Fig. 2A, arrow) before invading. [The two invasion events shown in Figs. 1 and 2 can be viewed as videos in the supplementary material on the PNAS web site (see www.pnas.org)].
Figure 1.
DIC images of the invasion of a P. gallinaceum ookinete into an A. aegypti midgut cell. The times are given relative to the start of invasion. The scale bar in G is for images A–G; image H is reduced in magnification to show the difference in refractive index of invaded cells relative to neighboring cells. (A) T = −48 sec. (B) T = −15 sec. The ookinete is gliding on the surface of the cell to be invaded. (C) T = 0 sec. Invasion into the lateral apical membrane of the midgut cell has begun and the anterior end of the ookinete has disappeared beneath the cell surface. (D) T = 7 sec. Invasion is near completion, and only the posterior end of the ookinete is visible on the midgut cell surface. (E) T = 2.8 min. The ookinete (arrow) is barely visible inside the invaded cell. (F) T = 3.4 min. The ookinete (arrow) invades a neighboring cell. (G) T = 5.0 min. The ookinete (arrow) is entirely within the neighboring cell. (Inset) Polarized light microscopy unambiguously identifies the position of the ookinete on the basis of the birefringent hemozoin pigment granules. (H) T = 11.3 min. The change in surface morphology and decrease in refractive index of invaded cells are hallmarks of the invasion process.
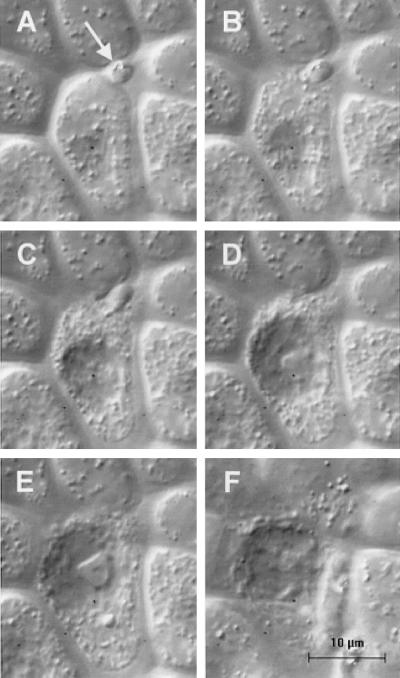
Figure 2.
Rapid morphological change and death of a midgut cell during invasion. The magnification for all six images is identical. The focal plane for images A–D is identical; the focal plane for image E is slightly lower and for image F slightly higher than for images A–D. (A) T = 0. An ookinete is positioned between the apical free margins of two midgut cells with only the posterior end visible (arrow). The focal plane of the image is slightly below the cell surface. (B) T = 8 sec. The midgut cell is beginning to disintegrate. (C) T = 17 sec. The cell continues to disintegrate and cell contents are being discharged. The cell's nucleus has become more prominent. (D) T = 32 sec. The ookinete is completely interiorized within the midgut cell and has disappeared from view. (E) T = 57 sec. The midgut cell's morphology is grossly altered. (F) T = 1.4 min. A surface view of the invaded cell showing the marked decrease in refractive index of the cell.
Invasion of midgut cells is rapid; measured from the first visible sign of entry of a midgut cell, the ookinete is completely interiorized in an average of 1.3 min (Table 1). However, ookinetes do not always invade midgut cells. We observed instances where an ookinete was on the midgut surface or between the apical surfaces of two midgut cells, but no invasion or further movement occurred during the time that we were able to observe the parasite (up to 1.5 h after the initial movement of the parasite onto the midgut cell surface).
After invasion, the ookinetes are difficult to visualize by DIC microscopy. However, as the hemozoin pigment granules in the parasite are birefringent, we could follow the intracellular movement of the ookinetes by using polarized light microscopy (Fig. 1G Inset). Once intracellular, the ookinetes migrate basolaterally toward neighboring midgut cells (Fig. 1E, arrow) and toward the midgut basement membrane. On 7 of 19 occasions, we observed an ookinete crossing into one or more midgut cells adjacent to the originally invaded cell (Fig. 1 F and G, arrows). In one instance, six midgut cells were invaded successively.
A hallmark of the invasion process is the rapid appearance of marked morphological changes in the midgut cell. Without exception, the refractive index of invaded cells decreases in comparison to neighboring cells. This process takes an average of 1.1 min from the onset of invasion (Table 1). In some cases, the refractive index of the midgut cell changes before the onset of penetration, whereas the ookinete is still gliding over the cell surface, suggesting that surface contact with the ookinete is sufficient to initiate this change. The decrease in refractive index is observable at the cell surface (Fig. 1H) as well as at lower focal planes (Fig. 1E) and is always followed by a visible deterioration of the cell, as evidenced by nuclear swelling, movement of the nucleus to a more apical position in the cell, blebbing of the cell surface, and Brownian motion of particles within the cell. Occasionally, the morphological changes of the midgut cell are so rapid that the cell appears to explode, releasing cytoplasmic contents in the process (Fig. 2).
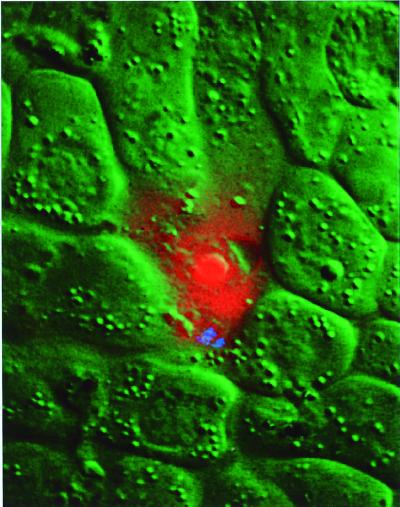
To confirm the premise that invaded midgut cells die in response to ookinete invasion, we observed ookinetes moving on the midgut surface in the presence of ethidium homodimer, a cell-impermeant fluorescent dye. Entry of the dye into an invaded cell is a sign of a ruptured plasma membrane and cell death (24, 25). We observed a perfect correlation between the presence of an ookinete inside a midgut cell and an intense fluorescent staining of the invaded cell compared with its neighbors (15 invaded midgut cells observed by fluorescence). Fig. 3 shows a dead midgut cell stained with ethidium homodimer (red). By DIC microscopy (green), the invaded cell exhibits a lower refractive index than its neighbors. The presence of an ookinete within the cell is confirmed by the presence of birefringent hemozoin pigment granules (blue). Similar results were obtained with the dyes propidium iodide and YO-PRO-1 (unpublished results).
Figure 3.
Composite color image of an ookinete inside a midgut cell showing ethidium homodimer staining of the invaded cell. The DIC image of the cell is shown in green, the ethidium homodimer fluorescence is shown in red, and the hemozoin pigment granules of the ookinete are shown in blue.
Death of midgut cells after invasion by ookinetes is also supported by previously published TEM images (cf. figure 2 in ref. 26). Invaded cells are electron lucent and heavily vacuolated and appear to lack an intact apical plasma membrane.
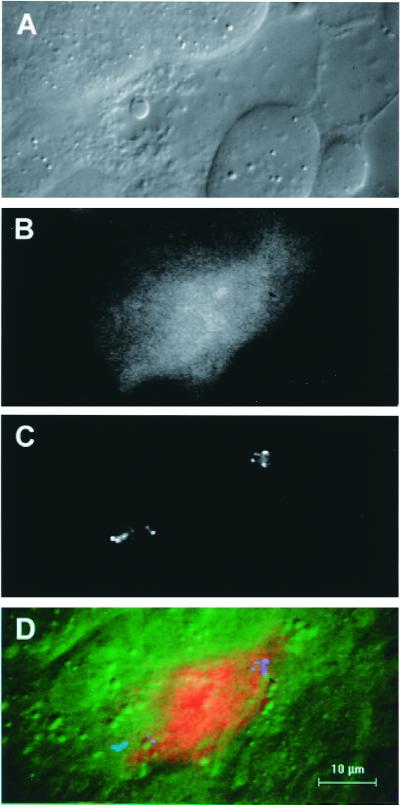
To determine whether death of the midgut cell is accompanied by activation of apoptosis-specific proteases, we preloaded midgut cells with PhiPhiLux-G1D2, a cell-permeant reagent that contains a caspase-3 consensus cleavage site and that increases in fluorescence intensity on proteolytic cleavage. With this dye, caspase activation can be observed in live cells. After preloading, excess dye was washed away, ookinetes were bound to the midguts, and invasion was allowed to proceed. We observed seven midgut cells with ookinetes inside them, and in each case, the fluorescent intensity of the invaded cell was higher than that of neighboring cells (Fig. 4). These data demonstrate that a caspase-3-specific protease is activated in midgut cells as a consequence of ookinete invasion. Caspase-3 activation of mammalian cells implies an irreversible commitment to apoptotic cell death (22, 27); a caspase with the same specificity has been found in insect cells (28). Therefore, it is likely that an apoptosis cascade is initiated in ookinete-invaded midgut cells.
Figure 4.
Images of an invaded cell stained with PhiPhiLux-G1D2. (A) DIC image of the invaded cell. (B) Increased fluorescence of the invaded cell compared with its neighbors. (C) Polarized light microscope image of hemozoin pigment granules of two ookinetes in the invaded cell. (D) Composite color image with DIC in green, fluorescence in red, and ookinete position in blue, showing the overall relationship between these three independent parameters.
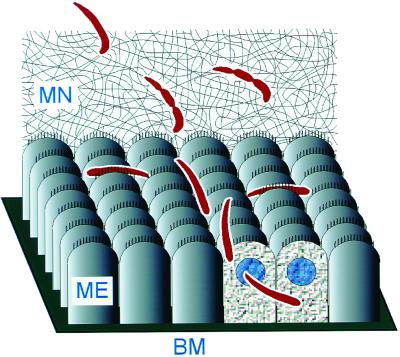
The mechanism of midgut invasion is not specific to P. gallinaceum ookinetes invading A. aegypti midgut cells; it occurs identically with other malaria species invading the midguts of anopheline mosquitoes. The invasion process of ookinetes of the rodent malaria parasite P. berghei into the midgut epithelium of A. stephensi was identical to invasion events observed with the avian malaria parasite P. gallinaceum and A. aegypti (unpublished results). Therefore, we propose that the process by which malaria parasites penetrate the midgut cells of their respective mosquito hosts is general and conserved. The main features of this process are shown schematically in Fig. 5.
Figure 5.
Schematic diagram of ookinete movement and midgut invasion. The ookinete first contacts the MN, through which it must navigate to reach the midgut surface. In the process of migrating through the MN, the ookinete develops constrictions along its length. Once on the surface of the midgut epithelium (ME), the parasite glides rapidly and tends to stay in the crevices between cells. Invasion occurs at the lateral apical membrane of a midgut cell. During its descent toward the basement membrane (BM), the ookinete often crosses to a neighboring cell. The refractive index of the invaded cells decreases, and their nuclei become more visible.
Discussion
We have created an in vitro system that allows detailed observations of ookinete movement on the midgut surface, and of ookinete invasion of midgut cells. Whenever in vitro models are used to study a complex biological process, the question arises of how closely the in vitro observations correspond to events in vivo. Although this is impossible to determine with certainty, our observations are in agreement with previous studies of ookinete behavior and the effect of ookinete invasion on the midgut. The movement of the parasite, including the formation of constrictions, corresponds to that observed in an ex vivo system (freshly dissected infected mosquitoes) in a previous study (9). Furthermore, the killing of midgut cells by invading ookinetes is consistent with previously reported deleterious effects of Plasmodium infection on mosquito midguts, including epithelial cell damage (29, 30) and induction of the mosquito's innate immune response (31). As a result, we believe that our in vitro observations closely mirror the events occurring during ookinete infections of midguts in vivo, although it is possible that the kinetics of ookinete migration and invasion are slightly different in vivo.
In previous TEM studies of midgut invasion by the human malaria parasite P. falciparum as well as other malaria parasites, the ookinetes were found both within and between midgut cells (3–8). It has been proposed that the ookinete leaves the midgut cell after invasion and continues its migration intercellularly toward the basement membrane (8). All of our observations as well as a previous study (26) clearly show an intracellular route of invasion. In addition, we have demonstrated that the ookinetes invariably invade specifically through the lateral apical plasma membrane of midgut cells and that this process is very rapid. Although we were not able to determine where the ookinete exits the invaded cell through the basolateral plasma membrane, we clearly demonstrate that ookinetes move from one midgut cell to another.
Our observations indicate that an apoptosis cascade is initiated in ookinete-infected midgut cells. Although midgut cell death in some cases is too rapid to be apoptotic (Fig. 2), apoptotic cell death may occur in other invaded cells, where cell death is slower (Fig. 1). The methods we used in the experiments with PhiPhiLux-G1D2 and ethidium homodimer did not allow us to determine precisely when caspase activation and cell death occur relative to either the completion of invasion, or to the refractive index changes in the invaded cell. With both dyes, we observed heightened fluorescence of invaded cells within 30–90 min of the first contact between ookinetes and midgut epithelia.
The rapid kinetics of caspase activation in invaded midgut cells is consistent with observations on caspase activation in other systems. Huston et al. detected caspase activation within 1–4 min after the interaction of Jurkat cells with Entamoeba histolytica (32). In studies of apoptosis induced by microinjection of cytochrome c into NRK-52E rat kidney tubule epithelial cells, the first signs of cell death were observed at 30 min after the induction of apoptosis, and the changes taking place in the apoptotic cells were described as “explosive” (33).
The death of host cells as a consequence of invasion by a pathogen is not without precedent. For example, pathogenic intracellular bacteria kill mammalian macrophages and M cells (34, 35); in the case of the macrophages, this host cell death is apoptotic (36, 37). The induction of apoptosis in invaded midgut cells may be a defense mechanism of the mosquito designed to inactivate the parasite (38) or to stimulate the mosquito's innate immune response (31). This reaction may contribute to the 70-fold attrition of ookinetes between the midgut lumen and the formation of oocysts (39). However, it is also possible that the refractive index changes and the activation of caspases in the invaded cell positively contribute to the successful entry of the ookinete into the midgut cell. To test whether caspase activation influences parasite development, we are planning to express insect-specific caspase inhibitors (IAP proteins) in the midgut of A. aegypti.
The death of mosquito midgut cells after invasion by an ookinete has additional implications in that it obligates us to reexamine previous reports proposing a unique cell type, the “Ross cell” (26), as a specific target of malaria ookinetes. We found no evidence to support the existence of a morphologically distinct midgut cell type invaded preferentially by ookinetes; the invaded cells observed in our study are indistinguishable from other midgut cells before invasion. Furthermore, recent TEM studies have failed to show the presence of cells in intact uninfected bloodfed midguts with the characteristics of “Ross cells” (H.Z. & E. R. Fischer, unpublished results). Therefore, the drastic morphological changes in midgut cells after invasion make it likely that some of the unique physical characteristics attributed to “Ross cells” are in fact a consequence of, rather than a prerequisite for, invasion.
In light of our observations that ookinetes frequently cross from within one midgut cell to another, the previous observation that ookinete-invaded midgut cells express a high level of a vesicular ATPase marker (26, 40) also makes it unlikely that a unique cell type is involved in invasion. To be consistent with the current and previous data, ookinetes would have to move from one v-ATPase-overexpressing cell to another. However, these cells represent a small percentage of the total midgut cell population and are found scattered in the posterior part of the midgut (40). We consider it more likely that v-ATPase overexpression is another consequence of midgut cell invasion by the ookinete. Interestingly, v-ATPase overexpression can delay or prevent apoptosis in human neutrophils or other mammalian cell types by preventing cytosolic acidification that precedes apoptosis (41–43). Overexpression of v-ATPase may thus be an adaptive response of the mosquito midgut cell to pH changes caused by the invading ookinete.
Given our observation that ookinetes can glide on the midgut surface for a long time before invasion, during which time a parasite may contact numerous midgut cells, it is possible that invasion is influenced by any of a number of systematic factors. These factors include the general health of the cell, its metabolic activity, and chemical or electrochemical gradients across its surface, or stochastic factors such as the density of microvilli or the accessibility of that particular cell's plasma membrane under the microvillar layer.
Ookinete invasion of mosquito midgut cells differs markedly from the mechanism of malaria merozoite invasion of red blood cells (11), or the invasion of various cell types by other apicomplexan parasites, all of which proceed via the formation of a transient or permanent parasitophorous vacuole [parasitophorus vacuole (PV), ref. 44]. In the process of parasitophorous vacuole formation during merozoite invasion of erythrocytes and toxoplasma invasion of nucleated vertebrate cells, a moving junction forms between the parasite and the host cell (45, 46). In contrast, ookinetes within midgut cells are clearly free in the cytoplasm (3, 4, 7, 8, 26). The lack of a PV in ookinete invasion may explain the lack of rhoptry structures in ookinetes (4, 47–50); rhoptries are associated with PV formation (51). The invasion mechanism used by ookinetes reflects the parasite's requirements at this stage of its life cycle: It must traverse the midgut epithelium to reach the basement membrane. The successful completion of this migration does not require the continued viability of the traversed midgut cell.
The in vitro system described here will allow us to study the process of ookinete invasion and oocyst development in greater detail and to test for the existence of chemoattractants that may activate the ookinete in the mosquito midgut and direct its movement to the midgut surface. It will also permit a determination of whether the refractoriness of nonpermissive species or strains of mosquitoes is a consequence of the inability of ookinetes to invade their midguts. The identification of mosquito factors that are absolutely required in the parasite's journey across the mosquito midgut may allow us to design specific insect vector-based inhibitors for the reduction of malaria transmission.
Supplementary Material
Acknowledgments
We thank Drs. Louis H. Miller and José M. C. Ribeiro for their support, for discussions of the work, and for their comments on the manuscript. We are grateful to Margery Sullivan (Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health) for her advice on P. berghei and for supplying us with P. berghei-infected mice. Thanks also to André Laughinghouse and Kevin Lee for technical help with mosquitoes and infected chickens. H.Z. was supported by the Vector Biology Network of the John D. and Catherine T. MacArthur Foundation. A video showing six invasion events is freely available from the authors.
Abbreviations
- MN
microvilli-associated network
- TEM
transmission electron microscopy
- DIC
differential interference contrast
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shortt H E. Trans R Soc Trop Med Hyg. 1948;42:227–230. doi: 10.1016/s0035-9203(48)80006-4. [DOI] [PubMed] [Google Scholar]
- 2.Stohler H. Acta Trop. 1957;14:302–352. [PubMed] [Google Scholar]
- 3.Garnham P C C, Bird R G, Baker J R. Trans R Soc Trop Med Hyg. 1962;56:116–120. doi: 10.1016/0035-9203(62)90137-2. [DOI] [PubMed] [Google Scholar]
- 4.Mehlhorn H, Peters W, Haberkorn A. Protistologica. 1980;16:135–154. [Google Scholar]
- 5.Meis J F, Ponnudurai T. Parasitol Res. 1987;73:500–506. doi: 10.1007/BF00535323. [DOI] [PubMed] [Google Scholar]
- 6.Meis J F, Pool G, van Gemert G J, Lensen A H, Ponnudurai T, Meuwissen J H. Parasitol Res. 1989;76:13–19. doi: 10.1007/BF00931065. [DOI] [PubMed] [Google Scholar]
- 7.Syafruddin, Arakawa R, Kamimura K, Kawamoto F. Parasitol Res. 1991;77:230–236. doi: 10.1007/BF00930863. [DOI] [PubMed] [Google Scholar]
- 8.Torii M, Nakamura K, Sieber K P, Miller L H, Aikawa M. J Protozool. 1992;39:449–454. doi: 10.1111/j.1550-7408.1992.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 9.Freyvogel T A. Acta Trop. 1966;23:201–222. [PubMed] [Google Scholar]
- 10.Yoeli, M. & Upmanis, R. S. (1968) Exp. Parasitol.22,. [DOI] [PubMed]
- 11.Dvorak J A, Miller L H, Whitehouse W C, Shiroishi T. Science. 1975;187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 12.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 13.Carter R, Gwadz R W, McAuliffe F M. Exp Parasitol. 1979;47:185–193. doi: 10.1016/0014-4894(79)90072-9. [DOI] [PubMed] [Google Scholar]
- 14.Warburg A, Miller L H. Science. 1992;255:448–450. doi: 10.1126/science.1734521. [DOI] [PubMed] [Google Scholar]
- 15.Trager W, Jensen J B. Int J Parasitol. 1997;27:989–1006. doi: 10.1016/s0020-7519(97)00080-5. [DOI] [PubMed] [Google Scholar]
- 16.Gerberg E J, Barnard D R, Ward R A. Manual for Mosquito Rearing and Experimental Techniques. Lake Charles, LA: American Mosquito Control Assoc.; 1994. [Google Scholar]
- 17.Higgs S, Beaty B J. In: The Biology of Disease Vectors. Beaty B J, Marquardt W C, editors. Niwot, CO: Univ. Press of Colorado; 1996. pp. 595–605. [Google Scholar]
- 18.Zieler H, Nawrocki J P, Shahabuddin M. J Exp Biol. 1999;202:485–495. doi: 10.1242/jeb.202.5.485. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal D C, Carter R, Howard R J, McAuliffe F M. Mol Biochem Parasitol. 1983;8:53–69. doi: 10.1016/0166-6851(83)90034-8. [DOI] [PubMed] [Google Scholar]
- 20.Munderloh U G, Kurtti T J. J Parasitol. 1987;73:919–923. [PubMed] [Google Scholar]
- 21.Zieler H, Garon C F, Fischer E R, Shahabuddin M. J Eukaryotic Microbiol. 1998;45:512–520. doi: 10.1111/j.1550-7408.1998.tb05110.x. [DOI] [PubMed] [Google Scholar]
- 22.Huppertz B, Frank H-G, Kaufmann P. Anat Embryol. 1999;200:1–18. doi: 10.1007/s004290050254. [DOI] [PubMed] [Google Scholar]
- 23.Zieler H, Garon C F, Fischer E R, Shahabuddin M. J Exp Biol. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]
- 24.Kaneshiro E S, Wyder M A, Wu Y-P, Cushion M T. J Microbiol Methods. 1993;17:1–16. [Google Scholar]
- 25.Papadopoulos N G, Dedoussis G V Z, Spanakos G, Gritzapis A D, Baxevanis C N, Papamichail M. J Immunol Methods. 1994;177:101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 26.Shahabuddin M, Pimenta P F. Proc Natl Acad Sci USA. 1998;95:3385–3389. doi: 10.1073/pnas.95.7.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zapata J M, Takahashi R, Salvesen G S, Reed J C. J Biol Chem. 1998;273:6916–6920. doi: 10.1074/jbc.273.12.6916. [DOI] [PubMed] [Google Scholar]
- 28.Dorstyn L, Read S H, Quinn L M, Richardson H, Kumar S. J Biol Chem. 1999;274:30778–30783. doi: 10.1074/jbc.274.43.30778. [DOI] [PubMed] [Google Scholar]
- 29.Seitz H M, Maier W A, Rottok M, Becker-Feldmann H. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1987;266:155–166. doi: 10.1016/s0176-6724(87)80029-9. [DOI] [PubMed] [Google Scholar]
- 30.Maier W A, Becker-Feldman H, Seitz H M. Parasitol Today. 1987;3:216–218. doi: 10.1016/0169-4758(87)90063-9. [DOI] [PubMed] [Google Scholar]
- 31.Dimopoulos G, Seeley D, Wolf A, Kafatos F C. EMBO J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huston, C. D., Lankford, M. F., Sandmeier, J. J., Hahn, C. S. & Petri, W. A. (1999) Mol. Biol. Cell10 Suppl., 1899.
- 33.Chang S H, Phelps P C, Berezesky I K, Ebersberger M L, Trump B F. Am J Pathol. 2000;156:637–649. doi: 10.1016/S0002-9440(10)64768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones B D, Ghori N, Falkow S. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinrauch Y, Zychlinsky A. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 36.Zychlinsky A, Prevost M C, Sansonsetti P J. Nature (London) 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 37.Monack D M, Raupach B, Hromockyj A E, Falkow S. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aliprantis A O, Diez-Roux G, Mulder L C F, Zychlinsky A, Lang R A. Immunol Today. 1996;17:573–576. doi: 10.1016/s0167-5699(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan J A, Noden B H, Beier J C. J Parasitol. 1992;78:716–724. [PubMed] [Google Scholar]
- 40.Cociancich S O, Park S S, Fidock D A, Shahabuddin M. J Biol Chem. 1999;274:12650–12655. doi: 10.1074/jbc.274.18.12650. [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb R A, Giensing H A, Zhu J Y, Engler R L, Babior B M. Proc Natl Acad Sci USA. 1995;92:5965–5968. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb R A, Gruol D L, Zhu J Y, Engler R L. J Clin Invest. 1996;97:2391–2398. doi: 10.1172/JCI118683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niessen H, Meisenholder G W, Li H L, Gluck S L, Lee B S, Bowman B, Engler R L, Babior B M, Gottlieb R A. Blood. 1997;90:4598–4601. [PubMed] [Google Scholar]
- 44.Lingelbach K, Joiner K. J Cell Sci. 1998;111:1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- 45.Aikawa M, Miller L H, Johnson J, Rabbege J. J Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michel R, Schupp K, Raether W, Bierther F W. Int J Parasitol. 1980;10:309–313. doi: 10.1016/0020-7519(80)90012-0. [DOI] [PubMed] [Google Scholar]
- 47.Garnham P C C, Bird R G, Baker J R, Desser S S, El-Nahal H M S. Trans R Soc Trop Med Hyg. 1969;63:187–194. doi: 10.1016/0035-9203(69)90145-x. [DOI] [PubMed] [Google Scholar]
- 48.Aikawa M. Exp Parasitol. 1971;30:284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- 49.Aikawa M, Carter R, Ito Y, Nijhout M M. J Protozool. 1984;31:403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 50.Sinden R E. In: Malaria: Parasite Biology, Pathogenesis and Protection. Sherman I W, editor. Washington, D.C.: Am. Soc. Microbiol.; 1998. pp. 25–48. [Google Scholar]
- 51.Dubremetz J F, Garcia-Reguet N, Conseil V, Fourmaux M N. Int J Parasitol. 1998;28:1007–1013. doi: 10.1016/s0020-7519(98)00076-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.