Abstract
BACKGROUND/AIMS—Pterygia are a common disorder of the ocular surface. The disease represents a chronic fibrovascular and degenerative process thought to originate at the conjunctival-corneal junction, where altered limbal stem cells are proposed to be the cell of origin. Extensive epidemiological evidence exists to implicate ultraviolet B irradiation in the pathogenesis of pterygia. To date no animal or in vitro culture model has been developed to test such an hypothesis. The aim of this study was to establish and characterise a pure population of epithelial cells derived from pterygium tissue. METHODS—Tissue specimens were obtained from patients undergoing pterygium excision. Explants were cultured in either serum free or serum supplemented medium. Primary and passaged cells were processed for light microscopy, analysed by flow cytometry, and characterised immunohistochemically using specific antibodies. RESULTS—In serum free culture, cuboidal cells with typical morphology of epithelial cells migrated from the pterygium explants from 3 days onwards and eventually formed a cohesive monolayer. Passaged cells consisted of 98.4% cytokeratin positive cells and demonstrated immunoreactivity for multiple cytokeratins, including AE1, AE3, AE5, but were negative for AE8. These cells also expressed an epithelial specific antigen, together with vimentin and mucin, as did epithelial cells in sections of pterygia. CONCLUSIONS—A relatively simple method of isolating pterygium epithelial cells has been established. Cultured pterygium epithelial cells are phenotypically and functionally similar to their in vivo counterparts with respect to keratin, vimentin, and mucin expression. In vitro assays using these cells may aid in elucidating the pathogenesis of pterygia.
Full Text
The Full Text of this article is available as a PDF (303.7 KB).
Figure 1 .
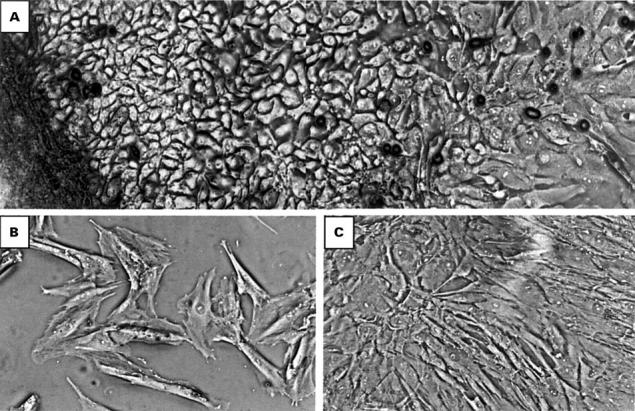
Phase contrast microscopy of primary and passaged human pterygium derived cells. (A) Primary explant culture of pterygium tissue (dark region, left on micrograph), grown in serum free media on collagen coated tissue culture plastic. Cell outgrowth was extensive, with cells displaying cuboidal morphology typical of epithelial cells. (B) Passaged cells were subcultured on uncoated surfaces in the presence of serum. Note the flattened morphology, similar to the cells observed at the leading growing edge in primary culture. (C) Confluent cells (cultured as in (B)) appear morphologically similar, suggesting a homogeneous population of cells. Cells in (B) and (C) were derived from passage 10 cultures and similar morphology was observed at earlier and later passages. All micrographs were taken at an original magnification of ×100.
Figure 2 .
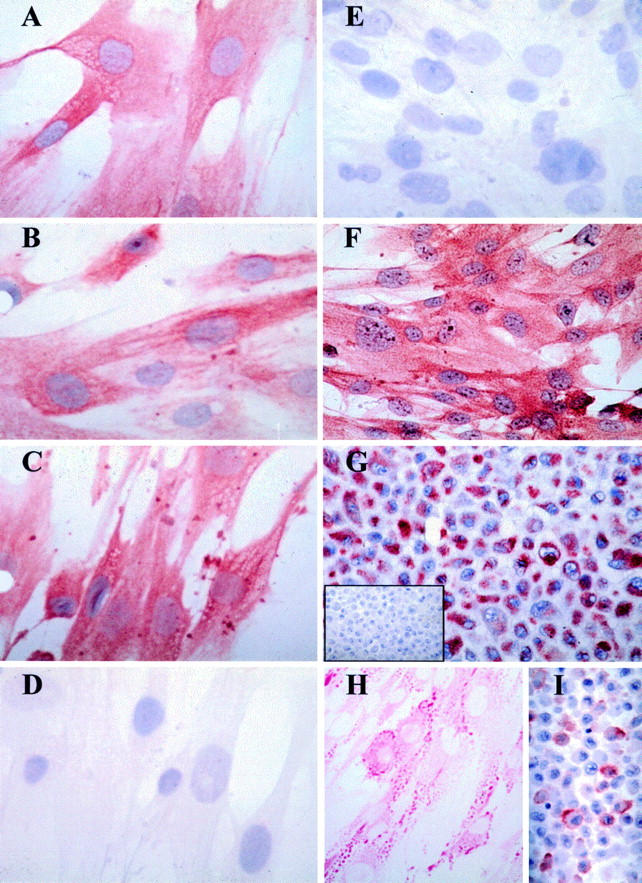
Histochemical characterisation of cultured pterygium derived epithelial cells. Pterygium derived cells were either cultured (passage 10) in chamber slides and fixed in methanol as mentioned in Materials and methods (A-F, and H) or cell pellet sections cut (G, I, and inset G) and used for histochemical analyses. Positive immunoreactive signal is denoted by red staining derived from the AEC chromogen. In all panels (except H) the cells were counterstained with haematoxylin. Cells incubated with the AE1 (A), AE5 (B), and AE3 (C) MoAbs demonstrated strong and specific cytokeratin immunoreactivity. Note the filamentous staining pattern observed with some MoAbs (B and C). No reactivity was observed with the AE8 Ab (D) or with an isotype control MoAb (E and inset G). All cultured cells uniformly expressed ESA (F), vimentin (G), stained positively with PAS (H), and were reactive to the MUC-1 MoAb (I). This staining pattern is representative of three separate experiments. Original magnification ×500.
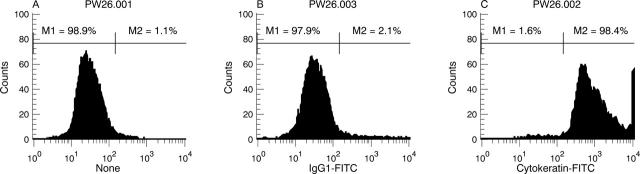
Figure 3 .
Flow cytometric analysis of cultured PEC. PEC at passage 10 were fluorescently labelled with a mouse anti-human pan-cytokeratin MoAb to determine the purity of cells in culture. The histograms generated indicate a homogeneous population of cultured cells (A), 98.4% of which are cytokeratin expressing (C). Minimal fluorescence was observed with a control Ab (B). Similar results were obtained on three other occasions using cells of earlier and later passages.
Figure 4 .
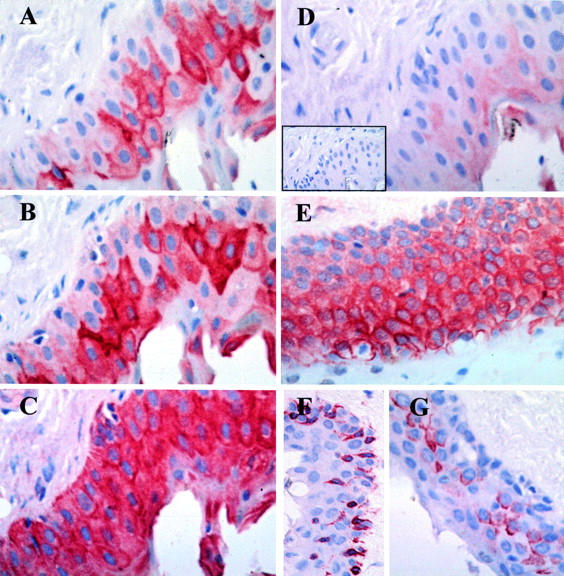
Immunohistochemical characterisation of epithelial cells in pterygia. Serial pterygium tissue sections (A-D and inset D) were incubated with the AE1 (A), AE5 (B), AE3 (C), AE8 (D) and an isotype control MoAb (inset D). Although the number of cells expressing the different keratins varied in the sections, some cells co-express AE1, AE3, and AE5. Very little or no reactivity was observed when tissue sections were stained with AE8 (D) or the isotype control MoAb (inset D). Abundant expression of ESA (E), vimentin (F), and mucin (G) was also noted in pterygium tissue, predominantly in the epithelium. Tissue sections were stained and counterstained as in Figure 2.These results are representative of all pterygium tissue studied. Original magnification ×640.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron M. E. Histology of pterygium: an electron microscopic study. Br J Ophthalmol. 1983 Sep;67(9):604–608. doi: 10.1136/bjo.67.9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo M. T., Müller-Stolzenburg N. W., Ho A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg. 1991 Dec;22(12):705–711. [PubMed] [Google Scholar]
- Coroneo M. T. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993 Nov;77(11):734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N., Tedla N., Lloyd A., Wakefield D. Expression of matrix metalloproteinases by human plasma cells and B lymphocytes. Eur J Immunol. 1998 Jun;28(6):1773–1784. doi: 10.1002/(SICI)1521-4141(199806)28:06<1773::AID-IMMU1773>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Diebold Y., Calonge M., Fernández N., Lázaro M. C., Callejo S., Herreras J. M., Pastor J. C. Characterization of epithelial primary cultures from human conjunctiva. Graefes Arch Clin Exp Ophthalmol. 1997 May;235(5):268–276. doi: 10.1007/BF01739635. [DOI] [PubMed] [Google Scholar]
- Dushku N., Reid T. W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994 Jul;13(7):473–481. doi: 10.3109/02713689408999878. [DOI] [PubMed] [Google Scholar]
- ELLIOTT R. The aetiology of pterygium. Trans Ophthalmol Soc N Z. 1961;13(Suppl):22–41. [PubMed] [Google Scholar]
- Hill J. C., Maske R. Pathogenesis of pterygium. Eye (Lond) 1989;3(Pt 2):218–226. doi: 10.1038/eye.1989.31. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Kiorpes T. C., Friend J., Thoft R. A. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):73–80. [PubMed] [Google Scholar]
- Kumar R. K., Maronese S. E., O'Grady R. Serum-free culture of mouse tracheal epithelial cells. Exp Lung Res. 1997 Sep-Oct;23(5):427–440. doi: 10.3109/01902149709039236. [DOI] [PubMed] [Google Scholar]
- Kwok L. S., Coroneo M. T. A model for pterygium formation. Cornea. 1994 May;13(3):219–224. doi: 10.1097/00003226-199405000-00005. [DOI] [PubMed] [Google Scholar]
- Landay A., Ohlsson-Wilhelm B., Giorgi J. V. Application of flow cytometry to the study of HIV infection. AIDS. 1990 Jun;4(6):479–497. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- Lauweryns B., van den Oord J. J., Missotten L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993 May;34(6):1991–1999. [PubMed] [Google Scholar]
- Levy W. J., Levy J., Clapper W. E. Pterygium tissue culture histoimmunological study. Arch Ophthalmol. 1970 Apr;83(4):402–405. doi: 10.1001/archopht.1970.00990030402003. [DOI] [PubMed] [Google Scholar]
- Lindberg K., Brown M. E., Chaves H. V., Kenyon K. R., Rheinwald J. G. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993 Aug;34(9):2672–2679. [PubMed] [Google Scholar]
- Pellegrini G., Traverso C. E., Franzi A. T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997 Apr 5;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Ratnakar K. S., Goswamy V., Agarwal L. P. Mast cells and pterygium. Acta Ophthalmol (Copenh) 1976 Jul;54(3):363–368. doi: 10.1111/j.1755-3768.1976.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Seifert P., Sekundo W. Capillaries in the epithelium of pterygium. Br J Ophthalmol. 1998 Jan;82(1):77–81. doi: 10.1136/bjo.82.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D. T., Lim A. S., Goh H. S., Smith D. R. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997 Mar;123(3):404–405. doi: 10.1016/s0002-9394(14)70141-2. [DOI] [PubMed] [Google Scholar]