Abstract
BACKGROUND/AIMS—Gelatinase B is a matrix metalloproteinase involved in extracellular matrix (ECM) breakdown often associated with scarring and other pathological disorders. It was investigated whether gelatinase B is involved in the pathogenesis of ECM degradation associated with trachomatous conjunctivitis. METHODS—Conjunctival biopsy specimens obtained from six patients with active trachoma, six patients with active vernal keratoconjunctivitis (VKC), and seven control subjects were studied. Immunohistochemical techniques and a specific monoclonal antibody against human gelatinase B were used, and a monoclonal antibody against macrophage CD68 to identify mononuclear cells with gelatinase B immunoreactivity. In addition, quantitative zymography was used to compare the activity of gelatinase B in conjunctival biopsy specimens from seven patients with active trachoma and seven control subjects. RESULTS—Gelatinase B was detected by immunohistochemistry only in polymorphonuclear cells located in the vascular lumens in three normal conjunctival biopsy specimens. In all trachoma specimens and in five VKC specimens, gelatinase B was localised in monocyte/macrophage cells, positive for the CD68 marker, and in polymorphonuclear cells. The majority of the latter cell type was located in intravascular spaces. Compared with VKC specimens, trachoma specimens showed significantly more immunoreactive gelatinase B monocyte/macrophage cells (52.3 (21.9) v 8.2 (6.4); p <0.001) and polymorphonuclear cells (23.2 (14.2) v 6.3 (5.4); p = 0.013). Activated macrophages with giant cell morphology clearly stained with the gelatinase B specific monoclonal antibody were observed in trachoma specimens. Zymography revealed that gelatinase B levels in trachoma specimens were significantly higher than the levels found in normal conjunctiva (1739.6 (1078.3) v 609.3 (395.9) scanning units; p = 0.0127). CONCLUSIONS—The increased activity of gelatinase B and numbers of inflammatory cells containing gelatinase B in trachoma specimens suggest that this enzyme plays a part in the pathogenesis of conjunctival scarring in trachoma.
Full Text
The Full Text of this article is available as a PDF (295.2 KB).
Figure 1 .
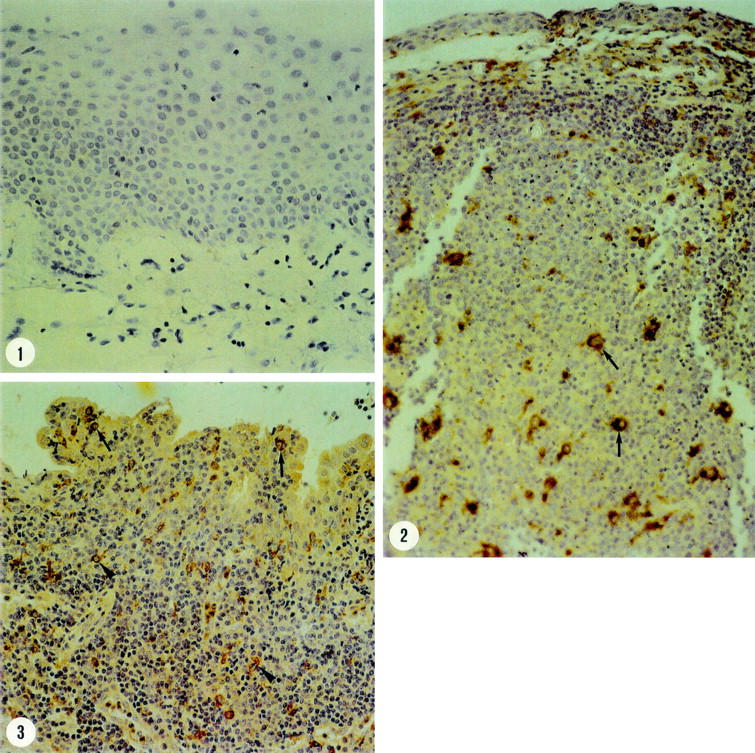
(1) Immunohistochemical staining for gelatinase B of conjunctiva from a normal control subject showing no immunoreactivity (three step avidin-peroxidase conjugated biotin complex; ×300). (2) Trachoma. Immunohistochemical staining for the macrophage marker CD68 showing immunoreactivity in the epithelial and stromal inflammatory infiltrate (arrows) (three step avidin-peroxidase conjugated biotin complex; ×300). (3) Trachoma. Immunohistochemical staining for gelatinase B showing immunoreactivity in the epithelial (arrows) and stromal (arrowheads) inflammatory infiltrate (three step avidin-peroxidase conjugated biotin complex; ×300).
Figure 2 .
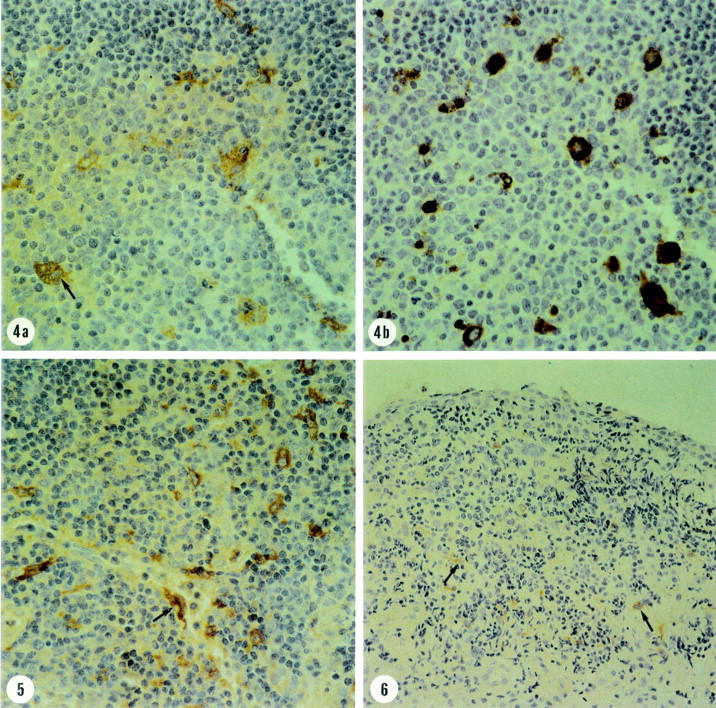
(4)Trachoma. Serial sections illustrating immunohistochemical stainings for gelatinase B (4a) and macrophage marker (CD68) (4b). Nearly all the macrophages are positive for gelatinase B. A multinucleated giant cell expresses gelatinase B (arrow) (three step avidin-peroxidase conjugated biotin complex; ×500). (5) Trachoma. Immunohistochemical staining for gelatinase B in monocyte/macrophage cell with dendritic morphology (arrow) (three step avidin-peroxidase conjugated biotin complex; ×500). (6) Vernal keratoconjunctivitis. Immunohistochemical staining for gelatinase B showing weak immunoreactivity in few monocytes/macrophages in the stromal inflammatory infiltrate (arrows) (three step avidin-peroxidase conjugated biotin complex; × 300).
Figure 3 .
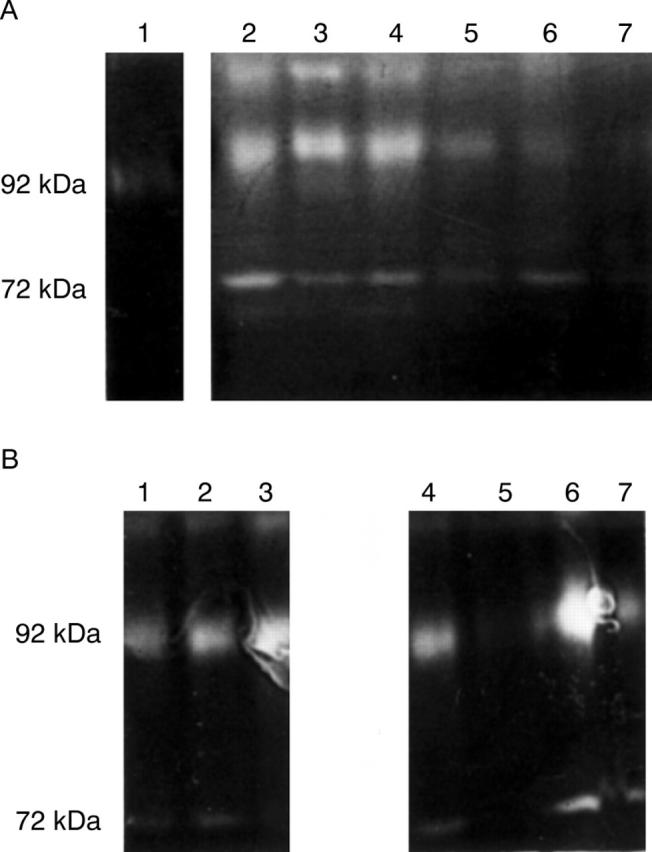
Gelatin zymography of conjunctival biopsy specimens from normal subjects (A), and from patients with active trachoma (B). The zymographies of these samples show the presence of both gelatinase A (72 kDa) and gelatinase B (92 kDa).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu el-Asrar A. M., Geboes K., Tabbara K. F., al-Kharashi S. A., Missotten L., Desmet V. Immunopathogenesis of conjunctival scarring in trachoma. Eye (Lond) 1998;12(Pt 3A):453–460. doi: 10.1038/eye.1998.104. [DOI] [PubMed] [Google Scholar]
- Abu el-Asrar A. M., Geboes K., al-Kharashi S. A., Tabbara K. F., Missotten L. Collagen content and types in trachomatous conjunctivitis. Eye (Lond) 1998;12(Pt 4):735–739. doi: 10.1038/eye.1998.179. [DOI] [PubMed] [Google Scholar]
- Abu el-Asrar A. M., Geboes K., al-Kharashi S. A., al-Mosallam A. A., Tabbara K. F., al-Rajhi A. A., Missotten L. An immunohistochemical study of collagens in trachoma and vernal keratoconjunctivitis. Eye (Lond) 1998;12(Pt 6):1001–1006. doi: 10.1038/eye.1998.257. [DOI] [PubMed] [Google Scholar]
- Brade L., Holst O., Kosma P., Zhang Y. X., Paulsen H., Krausse R., Brade H. Characterization of murine monoclonal and murine, rabbit, and human polyclonal antibodies against chlamydial lipopolysaccharide. Infect Immun. 1990 Jan;58(1):205–213. doi: 10.1128/iai.58.1.205-213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L., Hibbs M. S., Kearney M., Loushin C., Isner J. M. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995 Apr 15;91(8):2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Campbell S., Richmond S. J., Yates P. S., Storey C. C. Lipopolysaccharide in cells infected by Chlamydia trachomatis. Microbiology. 1994 Aug;140(Pt 8):1995–2002. doi: 10.1099/13500872-140-8-1995. [DOI] [PubMed] [Google Scholar]
- Cuzner M. L., Gveric D., Strand C., Loughlin A. J., Paemen L., Opdenakker G., Newcombe J. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996 Dec;55(12):1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Finlay G. A., O'Driscoll L. R., Russell K. J., D'Arcy E. M., Masterson J. B., FitzGerald M. X., O'Connor C. M. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997 Jul;156(1):240–247. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ishizaki M., Kudoh S., Kitaichi M., Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest. 1998 Jun;78(6):687–698. [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Grillet B., Dequeker J., Paemen L., Van Damme B., Opdenakker G. Gelatinase B in chronic synovitis: immunolocalization with a monoclonal antibody. Br J Rheumatol. 1997 Jul;36(7):744–747. doi: 10.1093/rheumatology/36.7.744. [DOI] [PubMed] [Google Scholar]
- Ingalls R. R., Rice P. A., Qureshi N., Takayama K., Lin J. S., Golenbock D. T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995 Aug;63(8):3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D., Waubant E., Galardy R., Bunnett N. W., Hauser S. L. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995 May 1;154(9):4379–4389. [PubMed] [Google Scholar]
- Magee D. M., Smith J. G., Bleicker C. A., Carter C. J., Bonewald L. F., Schachter J., Williams D. M. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992 Mar;60(3):1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone J. D., Richards M., Jeffrey J. J. Recruitment of peripheral mononuclear cells by mammalian collagenase digests of type I collagen. Matrix. 1991 Aug;11(4):289–295. doi: 10.1016/s0934-8832(11)80237-4. [DOI] [PubMed] [Google Scholar]
- Masure S., Proost P., Van Damme J., Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem. 1991 Jun 1;198(2):391–398. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McMillan W. D., Patterson B. K., Keen R. R., Shively V. P., Cipollone M., Pearce W. H. In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995 Aug;15(8):1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Nikkari S. T., Höyhtyä M., Isola J., Nikkari T. Macrophages contain 92-kd gelatinase (MMP-9) at the site of degenerated internal elastic lamina in temporal arteritis. Am J Pathol. 1996 Nov;149(5):1427–1433. [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol Today. 1994 Mar;15(3):103–107. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Opdenakker G., Van Damme J. Cytokines and proteases in invasive processes: molecular similarities between inflammation and cancer. Cytokine. 1992 Jul;4(4):251–258. doi: 10.1016/1043-4666(92)90064-x. [DOI] [PubMed] [Google Scholar]
- Paemen L., Martens E., Masure S., Opdenakker G. Monoclonal antibodies specific for natural human neutrophil gelatinase B used for affinity purification, quantitation by two-site ELISA and inhibition of enzymatic activity. Eur J Biochem. 1995 Dec 15;234(3):759–765. doi: 10.1111/j.1432-1033.1995.759_a.x. [DOI] [PubMed] [Google Scholar]
- Parshley D. E., Bradley J. M., Samples J. R., Van Buskirk E. M., Acott T. S. Early changes in matrix metalloproteinases and inhibitors after in vitro laser treatment to the trabecular meshwork. Curr Eye Res. 1995 Jul;14(7):537–544. doi: 10.3109/02713689508998400. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Schachter J., Lavrich P., Lipsitz E. C., Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989 Sep;57(9):2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Welgus H. G., Parks W. C. Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem. 1993 Aug 15;268(23):17354–17361. [PubMed] [Google Scholar]
- Sarén P., Welgus H. G., Kovanen P. T. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996 Nov 1;157(9):4159–4165. [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Tetlow L. C., Lees M., Ogata Y., Nagase H., Woolley D. E. Differential expression of gelatinase B (MMP-9) and stromelysin-1 (MMP-3) by rheumatoid synovial cells in vitro and in vivo. Rheumatol Int. 1993;13(2):53–59. doi: 10.1007/BF00307734. [DOI] [PubMed] [Google Scholar]
- Thompson R. W., Holmes D. R., Mertens R. A., Liao S., Botney M. D., Mecham R. P., Welgus H. G., Parks W. C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995 Jul;96(1):318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P., Houde M., Arbour N., Rochefort D., Masure S., Mandeville R., Opdenakker G., Oth D. Differential effects of PKC inhibitors on gelatinase B and interleukin 6 production in the mouse macrophage. Cytokine. 1995 Feb;7(2):130–136. doi: 10.1006/cyto.1995.1017. [DOI] [PubMed] [Google Scholar]
- Van Ranst M., Norga K., Masure S., Proost P., Vandekerckhove F., Auwerx J., Van Damme J., Opdenakker G. The cytokine-protease connection: identification of a 96-kD THP-1 gelatinase and regulation by interleukin-1 and cytokine inducers. Cytokine. 1991 May;3(3):231–239. doi: 10.1016/1043-4666(91)90021-5. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Bonewald L. F., Roodman G. D., Byrne G. I., Magee D. M., Schachter J. Tumor necrosis factor alpha is a cytotoxin induced by murine Chlamydia trachomatis infection. Infect Immun. 1989 May;57(5):1351–1355. doi: 10.1128/iai.57.5.1351-1355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Xie B., Dong Z., Fidler I. J. Regulatory mechanisms for the expression of type IV collagenases/gelatinases in murine macrophages. J Immunol. 1994 Apr 1;152(7):3637–3644. [PubMed] [Google Scholar]
- el-Asrar A. M., Emarah M. H., Van den Oord J. J., Geboes K., Desmet V., Missotten L. Conjunctival epithelial cells infected with Chlamydia trachomatis express HLA-DR antigens. Br J Ophthalmol. 1989 May;73(5):399–400. doi: 10.1136/bjo.73.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Asrar A. M., Van den Oord J. J., Geboes K., Missotten L., Emarah M. H., Desmet V. Immunopathology of trachomatous conjunctivitis. Br J Ophthalmol. 1989 Apr;73(4):276–282. doi: 10.1136/bjo.73.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]


