Abstract
BACKGROUND—Vasoproliferative tumours of the retina (VPTR) are benign tumours of unknown origin, occurring mostly in otherwise healthy patients. VPTR may be associated with other chorioretinal diseases, such as uveitis. The tumours, which histologically represent reactive gliovascular proliferations, are characterised by a pink to yellow appearance on funduscopy and are accompanied by exudative and haemorrhagic changes of the retina. METHODS—22 cases of VPTR in 21 patients were examined with a follow up period between 1 month and 6 years. Ophthalmological changes associated with VPTR were intraretinal and subretinal exudations (n=18), exudative detachments of the surrounding sensory retina (n=13), intraretinal and subretinal haemorrhages (n=10), exudative changes within the macula (n=10), hyperpigmentation of the retinal pigment epithelium at the border of the exudative retinal changes (n=9), and vitreous haemorrhages (n=4). Tumour biopsy was performed in two cases. Treatment consisted of plaque radiotherapy (n=14), plaque radiotherapy and cryotherapy (two), cryotherapy only (two), observation (three), and enucleation in one case of a blind and painful eye. RESULTS—Regression of the tumour and the associated exudative changes could be observed in all treated cases. Visual acuity at last follow up improved two lines or more in two cases, remained within two lines of the initial visual acuity in 15 cases, and worsened in the remaining five. Histopathological examination of the biopsy specimens and the tumour of the enucleated eye showed massive capillary proliferation with perivascular spindle-shaped glial cells of retinal origin. CONCLUSION—The correct diagnosis of VPTR is of importance as these lesions may lead to visual loss. Further, VPTR must be differentiated from angiomas associated with von Hippel-Lindau disease as well as from ocular and systemic malignancies. Regression of tumour thickness and associated retinal changes can be achieved with brachytherapy or cryotherapy.
Full Text
The Full Text of this article is available as a PDF (217.2 KB).
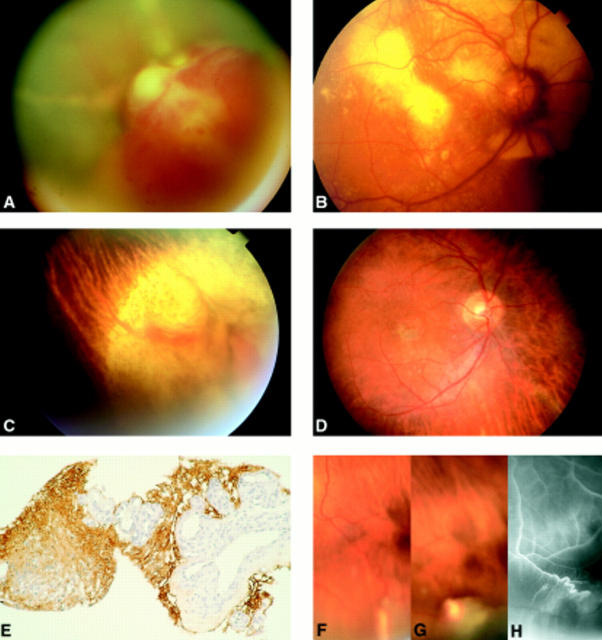
Figure 1 .
(A) VPTR in the lower retinal periphery in a 31 year old male patient who presented with floaters and loss of vision. The tumour (long arrow) is accompanied by an exudative retinal detachment, intraretinal accumulations of lipoid exudates, telangiectactic alterations of the retinal vessels (small arrow), and marked hyperpigmentations of the retinal pigment epithelium in the retina adjacent to the exudative detachment. (B) Symptomless VPTR in the lower retinal periphery in a 65 year old female patient. (C) Mid-phase fluorescein angiography of the tumour shown in (B) demonstrating the connection of tumour vessels with non-dilated retinal vessels. (D) Late phase fluorescein angiography of the tumour shown in (B) demonstrating preretinal leakage of dye on the tumour surface. (E) VPTR as shown in (B, C) 6 months after brachytherapy with 106-ruthenium. Regression of tumour thickness and exudative retinal changes. Chorioretinal scarring within the area that was covered by the 106-ruthenium plaque. (F) VPTR in the lower retinal periphery of a 15 year old boy with a history of toxoplasmosis retinochoroiditis. (G) Enucleation specimen with VPTR of the retinal periphery in a 27 year old male patient who presented with a blind and painful eye as a result of neovascular glaucoma. (H) Low power magnification of the retinal tumour demonstrating that it consists of a proliferation of glial cells and small blood vessels embedded in a hyalinised stromal matrix (haematoxylin and eosin stain, ×10 objective). (I) High power magnification of a hyalinised blood vessel surrounded by glial cells (haematoxylin and eosin stain, ×40 objective). (J) High power magnification of a blood vessel filled with erythrocytes and surrounded by glial cells and hyalinised stroma (Gomori stain, ×40 objective). (A and B were assembled using digitised fundus photographs and Adobe Photoshop software).
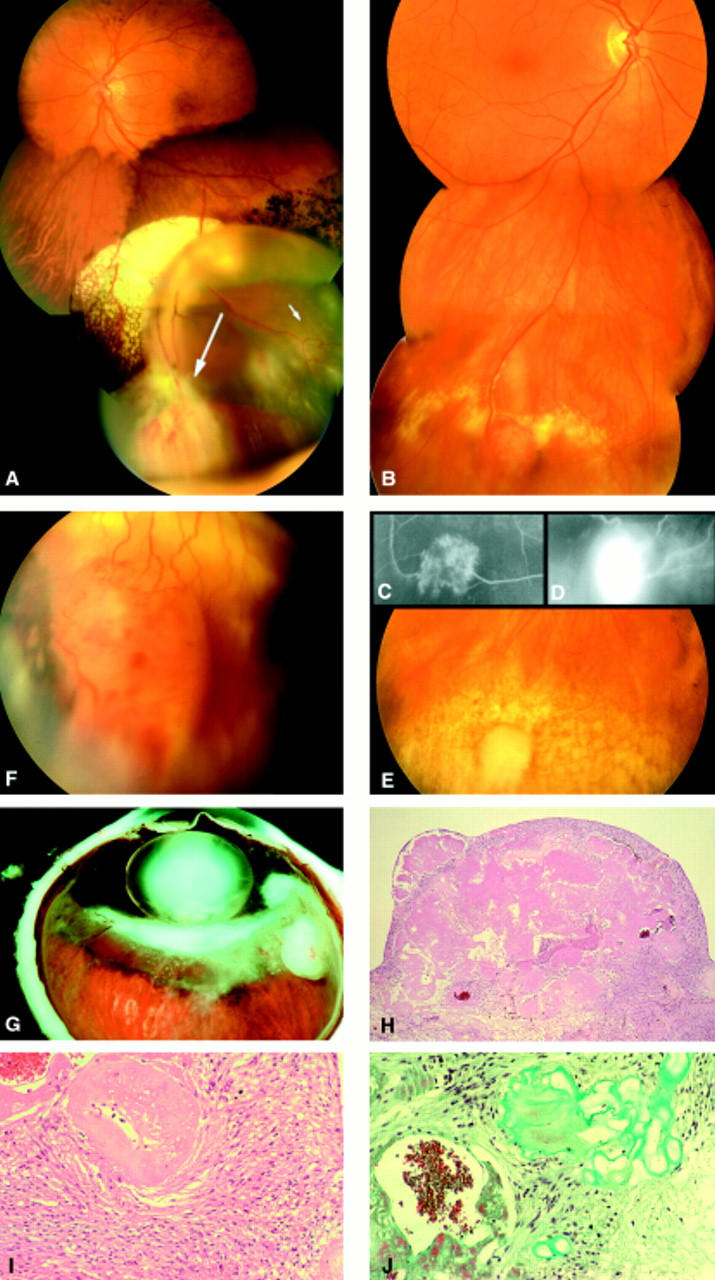
Figure 2 .
(A) VPTR of the lower nasal periphery of the right eye in a 31 year old male patient. Although the patient complained of visual loss and floaters for more than 6 months, diagnosis was delayed because no fundus examination had been performed by his ophthalmologist during this period. The tumour was biopsied following pars plana vitrectomy. (B) Massive intraretinal and subretinal exudations extending to the central retina in the same patient as (A), accompanied by retinal neovascularisations on the optic disc. (C) Same patient as in (A). Chorioretinal scar and complete regression of VPTR after 106-ruthenium brachytherapy. (D) Same patient as in (B). Complete regression of the central retinal exudates 1 year after 106-ruthenium brachytherapy. Note the residual changes of the RPE within the central macula. No significant gain in visual acuity could be noted despite regression of the exudative changes. (E) The biopsy specimen obtained from the tumour displayed in Figure 1 stained for glial fibrillary acid protein (GFAP, ×40 objective), demonstrating that the spindle cell population within the retinal tumours are of glial cell origin. The endothelium of the blood vessels is negative for this marker. (F) Left eye of the same patient as in (A). Initially, no fundus abnormalities could be noted in his fellow eye. After 1 year of follow up, a small VPTR could be seen in the lower retinal periphery of his fellow left eye. (G) Same tumour as in (F) demonstrating tumour growth after 4 weeks of follow up. The tumour was then treated with cryotherapy (picture taken with scleral indentation). (H) Fluorescein angiography of the same tumour as in (G) demonstrating the connections between the retinal vasculature and the tumour vessels.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augsburger J. J., Golden M. I., Shields J. A. Fluorescein angiography of choroidal malignant melanomas with retinal invasion. Retina. 1984 Fall-Winter;4(4):232–241. doi: 10.1097/00006982-198400440-00004. [DOI] [PubMed] [Google Scholar]
- Bardenstein D. S., Char D. H., Irvine A. R., Stone R. D. Extramacular disciform lesions simulating uveal tumors. Ophthalmology. 1992 Jun;99(6):944–951. doi: 10.1016/s0161-6420(92)31870-6. [DOI] [PubMed] [Google Scholar]
- Barr C. C., Rice T. A., Michels R. G. Angioma-like mass in a patient with retrolental fibroplasia. Am J Ophthalmol. 1980 May;89(5):647–650. doi: 10.1016/0002-9394(80)90281-0. [DOI] [PubMed] [Google Scholar]
- Berger B., Peyman G. A., Juarez C., Mason G., Raichand M. Massive retinal gliosis simulating choroidal melanoma. Can J Ophthalmol. 1979 Oct;14(4):285–290. [PubMed] [Google Scholar]
- Bloom S. M., Mahl C. F. Photocoagulation for serous detachment of the macula secondary to retinal astrocytoma. Retina. 1991;11(4):416–422. doi: 10.1097/00006982-199111040-00009. [DOI] [PubMed] [Google Scholar]
- Bovino J. A., Marcus D. F., Nelsen P. T. Fluorescein angiography in massive retinal gliosis. Am J Ophthalmol. 1987 Apr 15;103(4):593–593. doi: 10.1016/s0002-9394(14)74289-8. [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A., Conway B. P. Hemangiomalike masses of the retina. Arch Ophthalmol. 1988 Oct;106(10):1409–1413. doi: 10.1001/archopht.1988.01060140573025. [DOI] [PubMed] [Google Scholar]
- Cardoso R. D., Brockhurst R. J. Perforating diathermy coagulation for retinal angiomas. Arch Ophthalmol. 1976 Oct;94(10):1702–1715. doi: 10.1001/archopht.1976.03910040476003. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Daicker B. Melanosis retinae et papillae durch transretinal in den Glaskörper eingebrochenes malignes Aderhautmelanom. Ophthalmologica. 1973;166(6):460–471. doi: 10.1159/000306876. [DOI] [PubMed] [Google Scholar]
- Felder K. S., Brockhurst R. J. Neovascular fundus abnormalities in peripheral uveitis. Arch Ophthalmol. 1982 May;100(5):750–754. doi: 10.1001/archopht.1982.01030030754006. [DOI] [PubMed] [Google Scholar]
- Felder K. S., Brockhurst R. J. Retinal neovascularization complicating rhegmatogenous retinal detachment of long duration. Am J Ophthalmol. 1982 Jun;93(6):773–776. doi: 10.1016/0002-9394(82)90473-1. [DOI] [PubMed] [Google Scholar]
- Galinos S. O., Smith T. R., Brockhurst R. J. Angioma-like lesion in hemoglobin sickle cell disease. Ann Ophthalmol. 1979 Oct;11(10):1549–1552. [PubMed] [Google Scholar]
- Ganley J. P., Streeten B. W. Glial nodules of the inner retina. Am J Ophthalmol. 1971 May;71(5):1099–1103. doi: 10.1016/0002-9394(71)90583-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb F., Fammartino J. J., Stratford T. P., Brockhurst R. J. Retinal angiomatous mass. A complication of retinal detachment surgery. Retina. 1984 Summer-Fall;4(3):152–157. [PubMed] [Google Scholar]
- Green W. R. Bilateral Coats' disease. Massive gliosis of the retina. Arch Ophthalmol. 1967 Mar;77(3):378–383. doi: 10.1001/archopht.1967.00980020380016. [DOI] [PubMed] [Google Scholar]
- Haik B. G. Advanced Coats' disease. Trans Am Ophthalmol Soc. 1991;89:371–476. [PMC free article] [PubMed] [Google Scholar]
- Henkind P., Morgan G. Peripheral retinal angioma with exudative retinopathy in adults (coats's lesion). Br J Ophthalmol. 1966 Jan;50(1):2–11. doi: 10.1136/bjo.50.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobiec F. A., Brodie S. E., Haik B., Iwamoto T. Giant cell astrocytoma of the retina. A tumor of possible Mueller cell origin. Ophthalmology. 1983 Dec;90(12):1565–1576. doi: 10.1016/s0161-6420(83)34348-7. [DOI] [PubMed] [Google Scholar]
- Kaba F., el Baba F., Green W. R. Uveal neovascularization at the ora serrata and pars plana. Ann Ophthalmol. 1987 Mar;19(3):85–90. [PubMed] [Google Scholar]
- Kreusel K. M., Bornfeld N., Lommatzsch A., Wessing A., Foerster M. H. Ruthenium-106 brachytherapy for peripheral retinal capillary hemangioma. Ophthalmology. 1998 Aug;105(8):1386–1392. doi: 10.1016/S0161-6420(98)98017-4. [DOI] [PubMed] [Google Scholar]
- Lafaut B. A., Meire F. M., Leys A. M., Dralands G., De Laey J. J. Vasoproliferative retinal tumors associated with peripheral chorioretinal scars in presumed congenital toxoplasmosis. Graefes Arch Clin Exp Ophthalmol. 1999 Dec;237(12):1033–1038. doi: 10.1007/s004170050341. [DOI] [PubMed] [Google Scholar]
- Laqua H., Wessing A. Peripheral retinal telangiectasis in adults simulating a vascular tumor or melanoma. Ophthalmology. 1983 Nov;90(11):1284–1291. doi: 10.1016/s0161-6420(83)34390-6. [DOI] [PubMed] [Google Scholar]
- Leys A. M., Van Eyck L. M., Nuttin B. J., Pauwels P. A., Delabie J. M., Libert J. A. Metastatic carcinoma to the retina. Clinicopathologic findings in two cases. Arch Ophthalmol. 1990 Oct;108(10):1448–1452. doi: 10.1001/archopht.1990.01070120096036. [DOI] [PubMed] [Google Scholar]
- Madreperla S. A., Hungerford J. L., Plowman P. N., Laganowski H. C., Gregory P. T. Choroidal hemangiomas: visual and anatomic results of treatment by photocoagulation or radiation therapy. Ophthalmology. 1997 Nov;104(11):1773–1779. doi: 10.1016/s0161-6420(97)30027-x. [DOI] [PubMed] [Google Scholar]
- McCabe C. M., Mieler W. F. Six-year follow-up of an idiopathic retinal vasoproliferative tumor. Arch Ophthalmol. 1996 May;114(5):617–617. doi: 10.1001/archopht.1996.01100130609022. [DOI] [PubMed] [Google Scholar]
- McDonald H. R., Schatz H., Johnson R. N., Abrams G. W., Brown G. C., Brucker A. J., Han D. P., Lewis H., Mieler W. F., Meyers S. Vitrectomy in eyes with peripheral retinal angioma associated with traction macular detachment. Ophthalmology. 1996 Feb;103(2):329–335. doi: 10.1016/s0161-6420(96)30696-9. [DOI] [PubMed] [Google Scholar]
- Medlock R. D., Shields J. A., Shields C. L., Yarian D. L., Beyrer C. R. Retinal hemangioma-like lesions in eyes with retinitis pigmentosa. Retina. 1990;10(4):274–277. doi: 10.1097/00006982-199010000-00009. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Gragoudas E. S. Advances in treatment of retinal angiomas. Int Ophthalmol Clin. 1997 Fall;37(4):159–170. doi: 10.1097/00004397-199703740-00014. [DOI] [PubMed] [Google Scholar]
- Ramsay R. C., Kinyoun J. L., Hill C. W., Aturaliya U. P., Knobloch W. H. Retinal astrocytoma. Am J Ophthalmol. 1979 Jul;88(1):32–36. doi: 10.1016/0002-9394(79)90748-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Bardenstein D., Wiggert B., Lee L., Fletcher R. T., Chader G. Retinitis pigmentosa with segmental massive retinal gliosis. An immunohistochemical, biochemical, and ultrastructural study. Ophthalmology. 1987 Feb;94(2):180–186. doi: 10.1016/s0161-6420(87)33493-1. [DOI] [PubMed] [Google Scholar]
- Sahel J. A., Frederick A. R., Jr, Pesavento R., Albert D. M. Idiopathic retinal gliosis mimicking a choroidal melanoma. Retina. 1988;8(4):282–287. doi: 10.1097/00006982-198808040-00011. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg P., Meyer-Schwickerath G. Angiomatosis retinae im Alter. Ber Zusammenkunft Dtsch Ophthalmol Ges. 1977;74:205–208. [PubMed] [Google Scholar]
- Shields C. L., Shields J. A., Barrett J., De Potter P. Vasoproliferative tumors of the ocular fundus. Classification and clinical manifestations in 103 patients. Arch Ophthalmol. 1995 May;113(5):615–623. doi: 10.1001/archopht.1995.01100050083035. [DOI] [PubMed] [Google Scholar]
- Shields C. L., Shields J. A., Gündüz K., Freire J. E., Mercado G. Radiation therapy for uveal malignant melanoma. Ophthalmic Surg Lasers. 1998 May;29(5):397–409. [PubMed] [Google Scholar]
- Shields J. A., Decker W. L., Sanborn G. E., Augsburger J. J., Goldberg R. E. Presumed acquired retinal hemangiomas. Ophthalmology. 1983 Nov;90(11):1292–1300. doi: 10.1016/s0161-6420(83)34389-x. [DOI] [PubMed] [Google Scholar]
- Shields J. A., Decker W. L., Sanborn G. E., Augsburger J. J., Goldberg R. E. Presumed acquired retinal hemangiomas. Ophthalmology. 1983 Nov;90(11):1292–1300. doi: 10.1016/s0161-6420(83)34389-x. [DOI] [PubMed] [Google Scholar]
- Shields J. A., Joffe L., Guibor P. Choroidal melanoma clinically simulating a retinal angioma. Am J Ophthalmol. 1978 Jan;85(1):67–71. doi: 10.1016/s0002-9394(14)76667-x. [DOI] [PubMed] [Google Scholar]
- Smeets M. H., Mooy C. M., Baarsma G. S., Mertens D. E., Van Meurs J. C. Histopathology of a vasoproliferative tumor of the ocular fundus. Retina. 1998;18(5):470–472. doi: 10.1097/00006982-199805000-00016. [DOI] [PubMed] [Google Scholar]