Abstract
AIM—To examine epiretinal membranes of proliferative diabetic retinopathy (PDR) for the presence of selective matrix metalloproteinases (MMPs) and their natural inhibitors (TIMPs), in order to determine whether neovascularisation and fibrosis, characteristic of this complication of diabetes mellitus, are associated with specific anomalies of MMP or TIMP expression. METHODS—The presence of selected MMPs and TIMPs was investigated in 24 fibrovascular epiretinal membranes of PDR, and the findings compared with that observed in 21 avascular epiretinal membranes of proliferative vitreoretinopathy (PVR) and five normal retinas. Specimens were examined for deposition of interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), gelatinase A (MMP-2), gelatinase B (MMP-9), and three tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2, and TIMP-3). RESULTS—The results showed that unlike normal retina, which constitutively expresses MMP-1 and TIMP-2, a large proportion of PDR membranes (> 62%) stained for MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2, and TIMP-3. There were no differences in the expression of these molecules when compared with PVR membranes. A characteristic staining for MMP-9 was observed within the perivascular matrix of PDR membranes, and there was a significant increase in TIMP-2 expression by PDR membranes (p= 0.036) when compared with PVR membranes. CONCLUSIONS—The findings that MMPs involved in degradation of fibrovascular tissue matrix, as well as TIMP-1 and TIMP-2, are found in a large proportion of PDR membranes, and that their expression does not differ from that of PVR membranes, suggest the existence of common pathways of extracellular matrix degradation in pathological processes leading to retinal neovascularisation and fibrosis.
Full Text
The Full Text of this article is available as a PDF (184.0 KB).
Figure 1 .
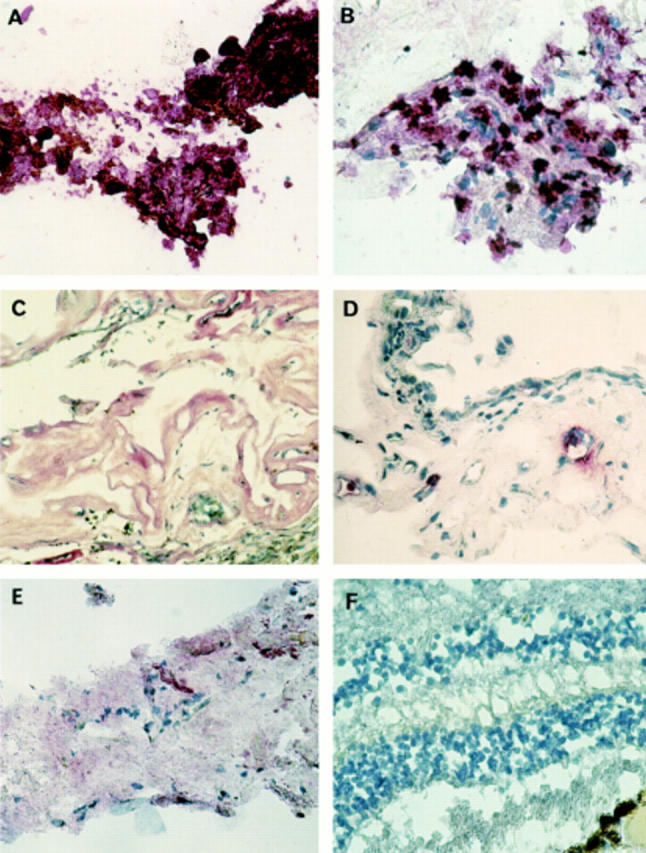
Photomicrographs of PDR membranes showing deposition of selective MMPs and TIMPs. The pink precipitate indicates the presence of these molecules within the membranes. Specimens were counterstained with Mayer's haematoxylin. (A) PDR membrane (No 15) showing strong staining (+++) for MMP-1. (B) PDR membrane (No 12) showing intense cellular and extracellular staining (+++) for MMP-2. (C) PDR membrane (No 1) exhibiting staining for MMP-9 within the extracellular and perivascular matrix. (D) Section of PDR membrane (No 17) showing detail staining for MMP-9 in the perivascular matrix. (E) PDR membrane exhibiting moderate staining (++) for TIMP-1 (No 11). (F) Normal retina showing negative staining for MMP-9.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Alexander J. P., Bradley J. M., Gabourel J. D., Acott T. S. Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990 Dec;31(12):2520–2528. [PubMed] [Google Scholar]
- Brown D., Hamdi H., Bahri S., Kenney M. C. Characterization of an endogenous metalloproteinase in human vitreous. Curr Eye Res. 1994 Sep;13(9):639–647. doi: 10.3109/02713689408999899. [DOI] [PubMed] [Google Scholar]
- De La Paz M. A., Itoh Y., Toth C. A., Nagase H. Matrix metalloproteinases and their inhibitors in human vitreous. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1256–1260. [PubMed] [Google Scholar]
- Dollery C. M., McEwan J. R., Henney A. M. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995 Nov;77(5):863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- Ebihara I., Nakamura T., Shimada N., Koide H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1998 Oct;32(4):544–550. doi: 10.1016/s0272-6386(98)70015-0. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Shafiee A., Schröder S., Knott R., McIntosh L. The role of growth factors in proliferative diabetic retinopathy. Eye (Lond) 1993;7(Pt 2):276–287. doi: 10.1038/eye.1993.61. [DOI] [PubMed] [Google Scholar]
- Franks W. A., Limb G. A., Stanford M. R., Ogilvie J., Wolstencroft R. A., Chignell A. H., Dumonde D. C. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11 (Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- Furness P. N. Basement membrane synthesis and degradation. J Pathol. 1997 Sep;183(1):1–3. doi: 10.1002/(SICI)1096-9896(199709)183:1<1::AID-PATH1096>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Garner A. Histopathology of diabetic retinopathy in man. Eye (Lond) 1993;7(Pt 2):250–253. doi: 10.1038/eye.1993.58. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Hiscott P., Unger W., Grierson I., McLeod D. Inflammation and the formation of epiretinal membranes. Eye (Lond) 1988;2 (Suppl):S140–S156. doi: 10.1038/eye.1988.140. [DOI] [PubMed] [Google Scholar]
- Gomez D. E., Alonso D. F., Yoshiji H., Thorgeirsson U. P. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997 Oct;74(2):111–122. [PubMed] [Google Scholar]
- Gottschall P. E., Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem. 1995 Apr;64(4):1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Grant M. B., Caballero S., Tarnuzzer R. W., Bass K. E., Ljubimov A. V., Spoerri P. E., Galardy R. E. Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes. 1998 Aug;47(8):1311–1317. doi: 10.2337/diab.47.8.1311. [DOI] [PubMed] [Google Scholar]
- Guo L., Hussain A. A., Limb G. A., Marshall J. Age-dependent variation in metalloproteinase activity of isolated human Bruch's membrane and choroid. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2676–2682. [PubMed] [Google Scholar]
- Hawrami K., Hitman G. A., Rema M., Snehalatha C., Viswanathan M., Ramachandran A., Mohan V. An association in non-insulin-dependent diabetes mellitus subjects between susceptibility to retinopathy and tumor necrosis factor polymorphism. Hum Immunol. 1996 Mar;46(1):49–54. doi: 10.1016/0198-8859(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Hiscott P., Waller H. A., Grierson I., Butler M. G., Scott D. L. The extracellular matrix of reparative tissue in the vitreous: fibronectin production in proliferative diabetic retinopathy membranes. Eye (Lond) 1993;7(Pt 2):288–292. doi: 10.1038/eye.1993.62. [DOI] [PubMed] [Google Scholar]
- Hiscott P., Waller H. A., Grierson I., Butler M. G., Scott D. L. The extracellular matrix of reparative tissue in the vitreous: fibronectin production in proliferative diabetic retinopathy membranes. Eye (Lond) 1993;7(Pt 2):288–292. doi: 10.1038/eye.1993.62. [DOI] [PubMed] [Google Scholar]
- Hunt R. C., Fox A., al Pakalnis V., Sigel M. M., Kosnosky W., Choudhury P., Black E. P. Cytokines cause cultured retinal pigment epithelial cells to secrete metalloproteinases and to contract collagen gels. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3179–3186. [PubMed] [Google Scholar]
- Jerdan J. A., Pepose J. S., Michels R. G., Hayashi H., de Bustros S., Sebag M., Glaser B. M. Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology. 1989 Jun;96(6):801–810. doi: 10.1016/s0161-6420(89)32818-1. [DOI] [PubMed] [Google Scholar]
- Kon C. H., Occleston N. L., Charteris D., Daniels J., Aylward G. W., Khaw P. T. A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1998 Jul;39(8):1524–1529. [PubMed] [Google Scholar]
- Kurizaki T., Toi M., Tominaga T. Relationship between matrix metalloproteinase expression and tumor angiogenesis in human breast carcinoma. Oncol Rep. 1998 May-Jun;5(3):673–677. doi: 10.3892/or.5.3.673. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Chignell A. H., Green W., LeRoy F., Dumonde D. C. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 1996 Feb;80(2):168–173. doi: 10.1136/bjo.80.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Pociot F., Briant L., Jongeneel C. V., Mölvig J., Worsaae H., Abbal M., Thomsen M., Nerup J., Cambon-Thomsen A. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993 Jan;23(1):224–231. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- Schwartz J. D., Monea S., Marcus S. G., Patel S., Eng K., Galloway A. C., Mignatti P., Shamamian P. Soluble factor(s) released from neutrophils activates endothelial cell matrix metalloproteinase-2. J Surg Res. 1998 Apr;76(1):79–85. doi: 10.1006/jsre.1998.5294. [DOI] [PubMed] [Google Scholar]
- Skyler J. S. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996 Jun;25(2):243–254. doi: 10.1016/s0889-8529(05)70323-6. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Thompson J. M., Weiss J. B. Matrix integrity and the control of angiogenesis. Int J Radiat Biol. 1991 Jul-Aug;60(1-2):61–64. doi: 10.1080/09553009114551551. [DOI] [PubMed] [Google Scholar]
- Webster L., Chignell A. H., Limb G. A. Predominance of MMP-1 and MMP-2 in epiretinal and subretinal membranes of proliferative vitreoretinopathy. Exp Eye Res. 1999 Jan;68(1):91–98. doi: 10.1006/exer.1998.0585. [DOI] [PubMed] [Google Scholar]


