Full Text
The Full Text of this article is available as a PDF (191.5 KB).
Figure 1 .
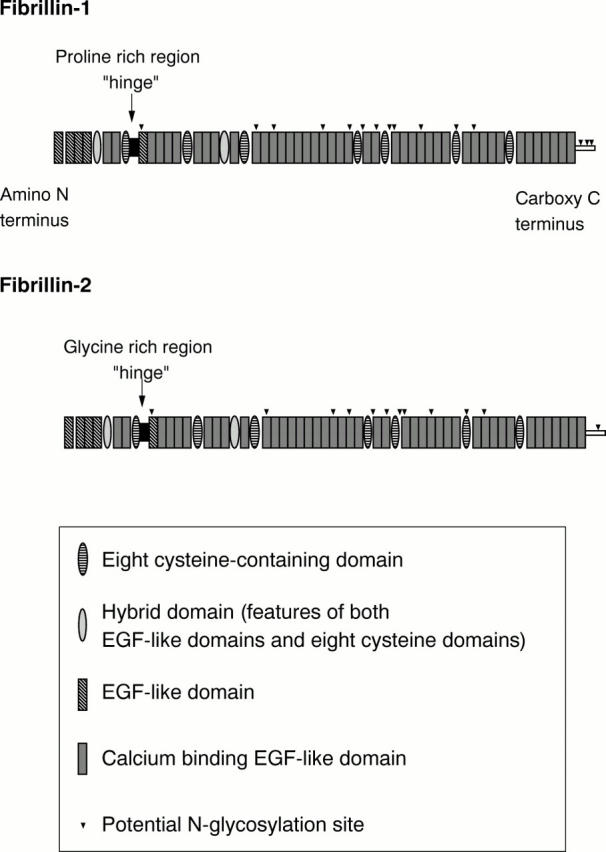
Schematic diagram of multidomain structure of fibrillin-1 and fibrillin-2.
Figure 2 .
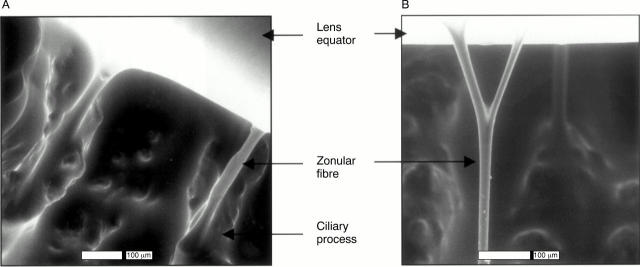
Environmental scanning electron microscopy of normal hydrated human zonules. (A) Zonular fibres arising from ciliary processes. (B) Branching of a zonular fibre.
Figure 3 .
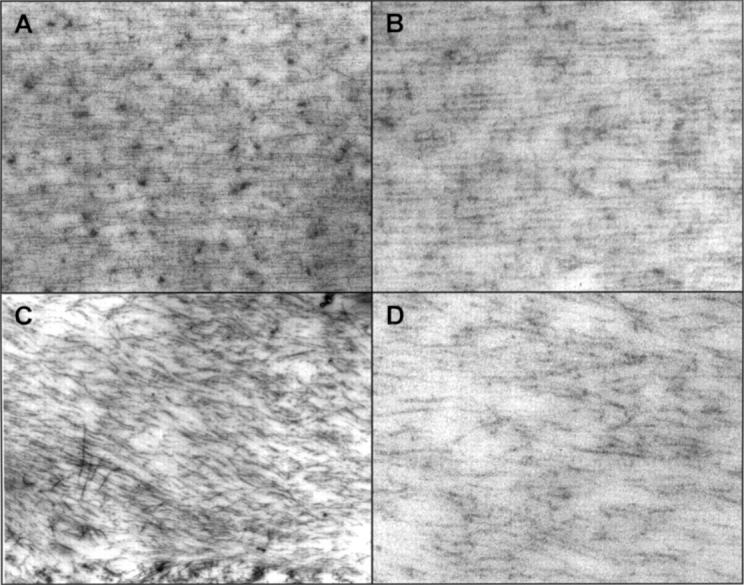
Transmission electron microscopy (TEM) of human zonular microfibrils, and effects of matrix metalloproteinase treatment. Normal human zonular specimens from a 54 year old were incubated for 3 hours at 37°C in the presence of 10 mM calcium chloride and hyaluronidase to remove adherent vitreous, with and without MMP-13, before fixation and TEM with uranyl acetate and lead citrate stain. (A) and (B) are untreated zonules which consist of dense and regular, parallel striated microfibrils. (C) and (D) are MMP-13 treated zonules. The zonular microfibrils are fragmented and irregularly and loosely arranged. Magnification: (A) and (C) ×20 000; (B) and (D) ×68 000.
Figure 4 .
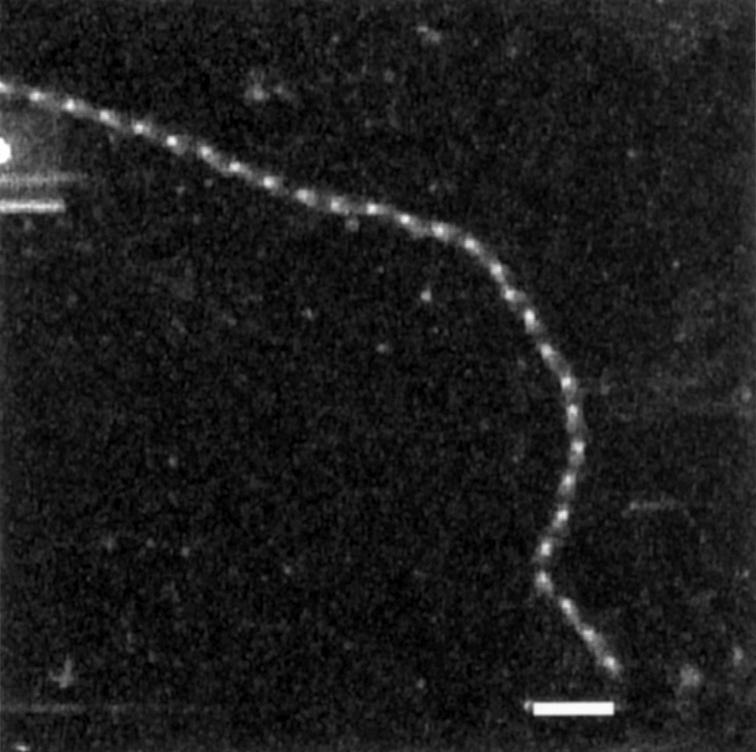
Scanning transmission electron microscopy of an isolated human zonular microfibril. Bar = 100 nm. Zonular microfibrils were biochemically isolated using an established method29 30 by incubation with collagenase and hyaluronidase in the presence of protease inhibitors, followed by size fractionation. The excluded volume (Vo) contained fibrillin-rich microfibrils.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu El-Asrar A. M., Dralands L., Veckeneer M., Geboes K., Missotten L., Van Aelst I., Opdenakker G. Gelatinase B in proliferative vitreoretinal disorders. Am J Ophthalmol. 1998 Jun;125(6):844–851. doi: 10.1016/s0002-9394(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Alexander J. P., Samples J. R., Acott T. S. Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr Eye Res. 1998 Mar;17(3):276–285. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- Alexander J. P., Samples J. R., Van Buskirk E. M., Acott T. S. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):172–180. [PubMed] [Google Scholar]
- Allen R. A., Straatsma B. R., Apt L., Hall M. O. Ocular manifestations of the Marfan syndrome. Trans Am Acad Ophthalmol Otolaryngol. 1967 Jan-Feb;71(1):18–38. [PubMed] [Google Scholar]
- Anand-Apte B., Pepper M. S., Voest E., Montesano R., Olsen B., Murphy G., Apte S. S., Zetter B. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci. 1997 Apr;38(5):817–823. [PubMed] [Google Scholar]
- Ando H., Twining S. S., Yue B. Y., Zhou X., Fini M. E., Kaiya T., Higginbotham E. J., Sugar J. MMPs and proteinase inhibitors in the human aqueous humor. Invest Ophthalmol Vis Sci. 1993 Dec;34(13):3541–3548. [PubMed] [Google Scholar]
- Ashworth J. L., Kelly V., Rock M. J., Shuttleworth C. A., Kielty C. M. Regulation of fibrillin carboxy-terminal furin processing by N-glycosylation, and association of amino- and carboxy-terminal sequences. J Cell Sci. 1999 Nov;112(Pt 22):4163–4171. doi: 10.1242/jcs.112.22.4163. [DOI] [PubMed] [Google Scholar]
- Ashworth J. L., Kelly V., Wilson R., Shuttleworth C. A., Kielty C. M. Fibrillin assembly: dimer formation mediated by amino-terminal sequences. J Cell Sci. 1999 Oct;112(Pt 20):3549–3558. doi: 10.1242/jcs.112.20.3549. [DOI] [PubMed] [Google Scholar]
- Ashworth J. L., Murphy G., Rock M. J., Sherratt M. J., Shapiro S. D., Shuttleworth C. A., Kielty C. M. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999 May 15;340(Pt 1):171–181. [PMC free article] [PubMed] [Google Scholar]
- Bishop P., Ayad S., Reardon A., McLeod D., Sheehan J., Kielty C. Type VI collagen is present in human and bovine vitreous. Graefes Arch Clin Exp Ophthalmol. 1996 Nov;234(11):710–713. doi: 10.1007/BF00292358. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Daga-Gordini D., Colombatti A., Castellani I., Marigo V., Volpin D. Emilin, a component of elastic fibers preferentially located at the elastin-microfibrils interface. J Cell Biol. 1993 Apr;121(1):201–212. doi: 10.1083/jcb.121.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. J., Bishop P., Hamdi H., Kenney M. C. Cleavage of structural components of mammalian vitreous by endogenous matrix metalloproteinase-2. Curr Eye Res. 1996 Apr;15(4):439–445. doi: 10.3109/02713689608995835. [DOI] [PubMed] [Google Scholar]
- Cross H. E., Jensen A. D. Ocular manifestations in the Marfan syndrome and homocystinuria. Am J Ophthalmol. 1973 Mar;75(3):405–420. doi: 10.1016/0002-9394(73)91149-5. [DOI] [PubMed] [Google Scholar]
- Das A., McGuire P. G., Eriqat C., Ober R. R., DeJuan E., Jr, Williams G. A., McLamore A., Biswas J., Johnson D. W. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999 Mar;40(3):809–813. [PubMed] [Google Scholar]
- Davis E. C., Mecham R. P. Intracellular trafficking of tropoelastin. Matrix Biol. 1998 Aug;17(4):245–254. doi: 10.1016/s0945-053x(98)90078-6. [DOI] [PubMed] [Google Scholar]
- De La Paz M. A., Itoh Y., Toth C. A., Nagase H. Matrix metalloproteinases and their inhibitors in human vitreous. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1256–1260. [PubMed] [Google Scholar]
- Downing A. K., Knott V., Werner J. M., Cardy C. M., Campbell I. D., Handford P. A. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996 May 17;85(4):597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- Faraco J., Bashir M., Rosenbloom J., Francke U. Characterization of the human gene for microfibril-associated glycoprotein (MFAP2), assignment to chromosome 1p36.1-p35, and linkage to D1S170. Genomics. 1995 Feb 10;25(3):630–637. doi: 10.1016/0888-7543(95)80004-6. [DOI] [PubMed] [Google Scholar]
- Farnsworth P. N., Burke P., Dotto M. E., Cinotti A. A. Ultrastructural abnormalities in a Marfan's syndrome lens. Arch Ophthalmol. 1977 Sep;95(9):1601–1606. doi: 10.1001/archopht.1977.04450090123010. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Girard M. T. Expression of collagenolytic/gelatinolytic metalloproteinases by normal cornea. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1779–1788. [PubMed] [Google Scholar]
- Fini M. E., Parks W. C., Rinehart W. B., Girard M. T., Matsubara M., Cook J. R., West-Mays J. A., Sadow P. M., Burgeson R. E., Jeffrey J. J. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996 Oct;149(4):1287–1302. [PMC free article] [PubMed] [Google Scholar]
- Freissler K., Küchle M., Naumann G. O. Spontaneous dislocation of the lens in pseudoexfoliation syndrome. Arch Ophthalmol. 1995 Sep;113(9):1095–1096. doi: 10.1001/archopht.1995.01100090017008. [DOI] [PubMed] [Google Scholar]
- Guggenheim J. A., McBrien N. A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1380–1395. [PubMed] [Google Scholar]
- Hanssen E., Franc S., Garrone R. Fibrillin-rich microfibrils: structural modifications during ageing in normal human zonule. J Submicrosc Cytol Pathol. 1998 Jul;30(3):365–369. [PubMed] [Google Scholar]
- Henderson M., Polewski R., Fanning J. C., Gibson M. A. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J Histochem Cytochem. 1996 Dec;44(12):1389–1397. doi: 10.1177/44.12.8985131. [DOI] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Smith R., Sykes B. C. A novel fibrillin mutation in the Marfan syndrome which could disrupt calcium binding of the epidermal growth factor-like module. Hum Mol Genet. 1993 Apr;2(4):475–477. doi: 10.1093/hmg/2.4.475. [DOI] [PubMed] [Google Scholar]
- Hindson V. J., Ashworth J. L., Rock M. J., Cunliffe S., Shuttleworth C. A., Kielty C. M. Fibrillin degradation by matrix metalloproteinases: identification of amino- and carboxy-terminal cleavage sites. FEBS Lett. 1999 Jun 11;452(3):195–198. doi: 10.1016/s0014-5793(99)00623-7. [DOI] [PubMed] [Google Scholar]
- Horrigan S. K., Rich C. B., Streeten B. W., Li Z. Y., Foster J. A. Characterization of an associated microfibril protein through recombinant DNA techniques. J Biol Chem. 1992 May 15;267(14):10087–10095. [PubMed] [Google Scholar]
- Huang S. H., Adamis A. P., Wiederschain D. G., Shima D. T., Shing Y., Moses M. A. Matrix metalloproteinases and their inhibitors in aqueous humor. Exp Eye Res. 1996 May;62(5):481–490. doi: 10.1006/exer.1996.0058. [DOI] [PubMed] [Google Scholar]
- Izquierdo N. J., Traboulsi E. I., Enger C., Maumenee I. H. Glaucoma in the Marfan syndrome. Trans Am Ophthalmol Soc. 1992;90:111–122. [PMC free article] [PubMed] [Google Scholar]
- Izquierdo N. J., Traboulsi E. I., Enger C., Maumenee I. H. Strabismus in the Marfan syndrome. Am J Ophthalmol. 1994 May 15;117(5):632–635. doi: 10.1016/s0002-9394(14)70069-8. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Karttunen L., Puhakka L., Sakai L., Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994 Jan;6(1):64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Saika S., Yamanaka O., Okada Y., Ohkawa K., Ohnishi Y. Immunolocalization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human subconjunctival tissues. Curr Eye Res. 1998 Apr;17(4):445–451. doi: 10.1080/02713689808951226. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Maddox B. K., Kuo H. J., Sakai L. Y., Glanville R. W. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991 Apr;39(4):441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- Kenney M. C., Chwa M., Alba A., Saghizadeh M., Huang Z. S., Brown D. J. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr Eye Res. 1998 Mar;17(3):238–246. doi: 10.1076/ceyr.17.3.238.5222. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Shuttleworth C. A. Fibrillin-containing microfibrils: structure and function in health and disease. Int J Biochem Cell Biol. 1995 Aug;27(8):747–760. doi: 10.1016/1357-2725(95)00028-n. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Shuttleworth C. A. Microfibrillar elements of the dermal matrix. Microsc Res Tech. 1997 Aug 15;38(4):413–427. doi: 10.1002/(SICI)1097-0029(19970815)38:4<413::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Shuttleworth C. A. Synthesis and assembly of fibrillin by fibroblasts and smooth muscle cells. J Cell Sci. 1993 Sep;106(Pt 1):167–173. doi: 10.1242/jcs.106.1.167. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Woolley D. E., Whittaker S. P., Shuttleworth C. A. Catabolism of intact fibrillin microfibrils by neutrophil elastase, chymotrypsin and trypsin. FEBS Lett. 1994 Aug 29;351(1):85–89. doi: 10.1016/0014-5793(94)00818-3. [DOI] [PubMed] [Google Scholar]
- Kon C. H., Occleston N. L., Charteris D., Daniels J., Aylward G. W., Khaw P. T. A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1998 Jul;39(8):1524–1529. [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Lönnqvist L., Child A., Kainulainen K., Davidson R., Puhakka L., Peltonen L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics. 1994 Feb;19(3):573–576. doi: 10.1006/geno.1994.1110. [DOI] [PubMed] [Google Scholar]
- Lönnqvist L., Reinhardt D., Sakai L., Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998 Dec;7(13):2039–2044. doi: 10.1093/hmg/7.13.2039. [DOI] [PubMed] [Google Scholar]
- Mariencheck M. C., Davis E. C., Zhang H., Ramirez F., Rosenbloom J., Gibson M. A., Parks W. C., Mecham R. P. Fibrillin-1 and fibrillin-2 show temporal and tissue-specific regulation of expression in developing elastic tissues. Connect Tissue Res. 1995;31(2):87–97. doi: 10.3109/03008209509028396. [DOI] [PubMed] [Google Scholar]
- McConnell C. J., DeMont M. E., Wright G. M. Microfibrils provide non-linear elastic behaviour in the abdominal artery of the lobster Homarus americanus. J Physiol. 1997 Mar 1;499(Pt 2):513–526. doi: 10.1113/jphysiol.1997.sp021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir S., Wheatley H. M., Hussels I. E., Whittum-Hudson J. A., Traboulsi E. I. A comparative histologic study of the fibrillin microfibrillar system in the lens capsule of normal subjects and subjects with Marfan syndrome. Invest Ophthalmol Vis Sci. 1998 Jan;39(1):84–93. [PubMed] [Google Scholar]
- Pereira L., D'Alessio M., Ramirez F., Lynch J. R., Sykes B., Pangilinan T., Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993 Jul;2(7):961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Pereira L., Lee S. Y., Gayraud B., Andrikopoulos K., Shapiro S. D., Bunton T., Biery N. J., Dietz H. C., Sakai L. Y., Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessier A. P., Potter K. A. Ocular pathology in bovine Marfan's syndrome with demonstration of altered fibrillin immunoreactivity in explanted ciliary body cells. Lab Invest. 1996 Jul;75(1):87–95. [PubMed] [Google Scholar]
- Plantner J. J., Smine A., Quinn T. A. Matrix metalloproteinases and metalloproteinase inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res. 1998 Feb;17(2):132–140. doi: 10.1076/ceyr.17.2.132.5610. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Qian R. Q., Glanville R. W. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry. 1997 Dec 16;36(50):15841–15847. doi: 10.1021/bi971036f. [DOI] [PubMed] [Google Scholar]
- Raghunath M., Putnam E. A., Ritty T., Hamstra D., Park E. S., Tschödrich-Rotter M., Peters R., Rehemtulla A., Milewicz D. M. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999 Apr;112(Pt 7):1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Pereira L. The fibrillins. Int J Biochem Cell Biol. 1999 Feb;31(2):255–259. doi: 10.1016/s1357-2725(98)00109-5. [DOI] [PubMed] [Google Scholar]
- Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest Ophthalmol. 1971 Nov;10(11):851–869. [PubMed] [Google Scholar]
- Reinhardt D. P., Keene D. R., Corson G. M., Pöschl E., Bächinger H. P., Gambee J. E., Sakai L. Y. Fibrillin-1: organization in microfibrils and structural properties. J Mol Biol. 1996 Apr 26;258(1):104–116. doi: 10.1006/jmbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- Reinhardt D. P., Mechling D. E., Boswell B. A., Keene D. R., Sakai L. Y., Bächinger H. P. Calcium determines the shape of fibrillin. J Biol Chem. 1997 Mar 14;272(11):7368–7373. doi: 10.1074/jbc.272.11.7368. [DOI] [PubMed] [Google Scholar]
- Reinhardt D. P., Ono R. N., Sakai L. Y. Calcium stabilizes fibrillin-1 against proteolytic degradation. J Biol Chem. 1997 Jan 10;272(2):1231–1236. doi: 10.1074/jbc.272.2.1231. [DOI] [PubMed] [Google Scholar]
- Ritty T. M., Broekelmann T., Tisdale C., Milewicz D. M., Mecham R. P. Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem. 1999 Mar 26;274(13):8933–8940. doi: 10.1074/jbc.274.13.8933. [DOI] [PubMed] [Google Scholar]
- Robinson P. N., Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet. 2000 Jan;37(1):9–25. doi: 10.1136/jmg.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J., Abrams W. R., Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993 Oct;7(13):1208–1218. [PubMed] [Google Scholar]
- Sakabe I., Oshika T., Lim S. J., Apple D. J. Anterior shift of zonular insertion onto the anterior surface of human crystalline lens with age. Ophthalmology. 1998 Feb;105(2):295–299. doi: 10.1016/s0161-6420(98)93172-4. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U., Naumann G. O. A histopathologic study of zonular instability in pseudoexfoliation syndrome. Am J Ophthalmol. 1994 Dec 15;118(6):730–743. doi: 10.1016/s0002-9394(14)72552-8. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Edwards J. H., Hall J. G. Mouse homologues of human hereditary disease. J Med Genet. 1994 Jan;31(1):1–19. doi: 10.1136/jmg.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond F, Trotter J. Morphology and biomechanics of the microfibrillar network of sea cucumber dermis. J Exp Biol. 1996;199(Pt 8):1817–1828. doi: 10.1242/jeb.199.8.1817. [DOI] [PubMed] [Google Scholar]
- Trask T. M., Ritty T. M., Broekelmann T., Tisdale C., Mecham R. P. N-terminal domains of fibrillin 1 and fibrillin 2 direct the formation of homodimers: a possible first step in microfibril assembly. Biochem J. 1999 Jun 15;340(Pt 3):693–701. [PMC free article] [PubMed] [Google Scholar]
- Vranka J. A., Johnson E., Zhu X., Shepardson A., Alexander J. P., Bradley J. M., Wirtz M. K., Weleber R. G., Klein M. L., Acott T. S. Discrete expression and distribution pattern of TIMP-3 in the human retina and choroid. Curr Eye Res. 1997 Feb;16(2):102–110. doi: 10.1076/ceyr.16.2.102.5086. [DOI] [PubMed] [Google Scholar]
- Wallace R. N., Streeten B. W., Hanna R. B. Rotary shadowing of elastic system microfibrils in the ocular zonule, vitreous, and ligamentum nuchae. Curr Eye Res. 1991 Jan;10(1):99–109. doi: 10.3109/02713689109007614. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994 Dec;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Wheatley H. M., Traboulsi E. I., Flowers B. E., Maumenee I. H., Azar D., Pyeritz R. E., Whittum-Hudson J. A. Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch Ophthalmol. 1995 Jan;113(1):103–109. doi: 10.1001/archopht.1995.01100010105028. [DOI] [PubMed] [Google Scholar]
- Wirtz M. K., Samples J. R., Kramer P. L., Rust K., Yount J., Acott T. S., Koler R. D., Cisler J., Jahed A., Gorlin R. J. Weill-Marchesani syndrome--possible linkage of the autosomal dominant form to 15q21.1. Am J Med Genet. 1996 Oct 2;65(1):68–75. doi: 10.1002/(SICI)1096-8628(19961002)65:1<68::AID-AJMG11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wright D. W., Mayne R. Vitreous humor of chicken contains two fibrillar systems: an analysis of their structure. J Ultrastruct Mol Struct Res. 1988 Sep;100(3):224–234. doi: 10.1016/0889-1605(88)90039-0. [DOI] [PubMed] [Google Scholar]
- Wright D. W., McDaniels C. N., Swasdison S., Accavitti M. A., Mayne P. M., Mayne R. Immunization with undenatured bovine zonular fibrils results in monoclonal antibodies to fibrillin. Matrix Biol. 1994 Jan;14(1):41–49. doi: 10.1016/0945-053x(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Ye H. Q., Azar D. T. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998 May;39(6):913–921. [PubMed] [Google Scholar]
- Zhang H., Apfelroth S. D., Hu W., Davis E. C., Sanguineti C., Bonadio J., Mecham R. P., Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994 Mar;124(5):855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Hu W., Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995 May;129(4):1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]