Abstract
AIM—To study the value of polymerase chain reaction (PCR) analysis, to detect viral DNA in recipient corneal buttons taken at the time of penetrating keratoplasty (PKP) in patients with an initial diagnosis of herpetic stromal keratitis (HSK). Since HSK has a tendency to recur, an accurate diagnosis of previous HSK could be the reason to start antiviral treatment immediately, thereby possibly decreasing the number of graft failures due to recurrent herpetic keratitis. METHODS—Recipient corneal buttons and aqueous humour (AH) samples were obtained at the time of PKP from HSK patients (n=31) and from other patients (n=78). Eye bank corneas were also used (n=23). Herpes simplex virus type 1 (HSV-1), type 2 (HSV-2), and varicella zoster virus (VZV) infection were assessed by PCR and antibody detection. RESULTS—The clinical diagnosis HSK could be confirmed by PCR for HSV-1 in 10/31 (32%). In these corneal buttons HSV-2 DNA was detected in 1/31 (3%) and VZV DNA in 6/31 (19%). Intraocular anti-HSV antibody production was detected in 9/28 AH samples tested (32%). In the other patient derived corneas HSV-1 DNA was detected in 13/78 (17%), including eight failed corneal grafts without clinically obvious herpetic keratitis in the medical history. In clear eye bank corneas HSV-1 was detected in 1/23 (4%). CONCLUSIONS—PCR of HSV-1 on corneal buttons can be a useful diagnostic tool in addition to detection of intraocular anti-HSV antibody production. Furthermore, the results were suggestive for the involvement of corneal HSV infection during allograft failure of corneas without previous clinical characteristic signs of herpetic keratitis.
Full Text
The Full Text of this article is available as a PDF (148.4 KB).
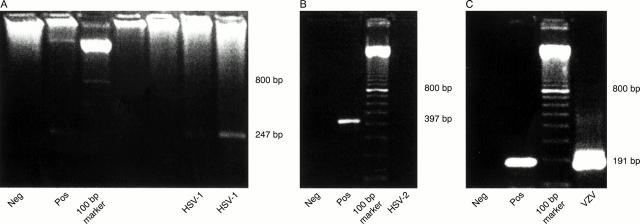
Figure 1 .
Electrophoresis gels of corneal DNA samples. The product sizes of the PCR for HSV-1, HSV-2, and VZV are indicated in (A), (B), and (C) respectively. Neg = negative control, Pos = positive control (explained in Materials and methods).
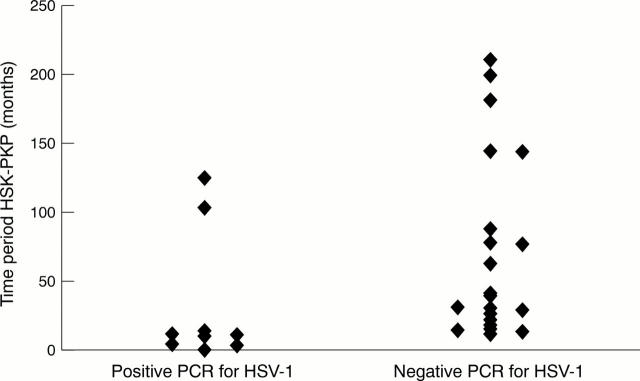
Figure 2 .
Cases with positive PCR results in the PCR for HSV-1 had significantly shorter time periods between the last episode of HSK and PKP then cases with negative PCRs (Mann-Whitney test, p=0.004). The median time period was 10 months (range 1-125 months) with positive HSV-1 PCRs and 41 months (range 11-210 months) with negative PCR results.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustamante C. I., Wade J. C. Herpes simplex virus infection in the immunocompromised cancer patient. J Clin Oncol. 1991 Oct;9(10):1903–1915. doi: 10.1200/JCO.1991.9.10.1903. [DOI] [PubMed] [Google Scholar]
- Cantin E. M., Chen J., McNeill J., Willey D. E., Openshaw H. Detection of herpes simplex virus DNA sequences in corneal transplant recipients by polymerase chain reaction assays. Curr Eye Res. 1991;10 (Suppl):15–21. doi: 10.3109/02713689109020353. [DOI] [PubMed] [Google Scholar]
- Cao M., Xiao X., Egbert B., Darragh T. M., Yen T. S. Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Invest Dermatol. 1989 Mar;92(3):391–392. doi: 10.1111/1523-1747.ep12277232. [DOI] [PubMed] [Google Scholar]
- Cleator G. M., Klapper P. E., Dennett C., Sullivan A. L., Bonshek R. E., Marcyniuk B., Tullo A. B. Corneal donor infection by herpes simplex virus: herpes simplex virus DNA in donor corneas. Cornea. 1994 Jul;13(4):294–304. doi: 10.1097/00003226-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Cook S. D. Herpes simplex virus in the eye. Br J Ophthalmol. 1992 Jun;76(6):365–366. doi: 10.1136/bjo.76.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster D. J. The Montgomery Lecture. Some factors which affect the visual outcome of corneal transplantation. Eye (Lond) 1991;5(Pt 3):265–278. doi: 10.1038/eye.1991.43. [DOI] [PubMed] [Google Scholar]
- Garweg J. G., Boehnke M. Low rate shedding of HSV-1 DNA, but not of infectious virus from human donor corneae into culture media. J Med Virol. 1997 Jul;52(3):320–325. doi: 10.1002/(sici)1096-9071(199707)52:3<320::aid-jmv14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Garweg J., Böhnke M. Slow viral replication of HSV-1 is responsible for early recurrence of herpetic keratitis after corneal grafting. Graefes Arch Clin Exp Ophthalmol. 1996 Aug;234 (Suppl 1):S133–S138. doi: 10.1007/BF02343062. [DOI] [PubMed] [Google Scholar]
- Holbach L. M., Font R. L., Baehr W., Pittler S. J. HSV antigens and HSV DNA in avascular and vascularized lesions of human herpes simplex keratitis. Curr Eye Res. 1991;10 (Suppl):63–68. doi: 10.3109/02713689109020359. [DOI] [PubMed] [Google Scholar]
- Kaye S. B., Lynas C., Patterson A., Risk J. M., McCarthy K., Hart C. A. Evidence for herpes simplex viral latency in the human cornea. Br J Ophthalmol. 1991 Apr;75(4):195–200. doi: 10.1136/bjo.75.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Larkin D. F. Corneal transplantation for herpes simplex keratitis. Br J Ophthalmol. 1998 Feb;82(2):107–108. doi: 10.1136/bjo.82.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Christianson M. D., Brady A. G. Ocular inoculation of monkeys with simian varicella virus: clinical and histopathologic observations. Invest Ophthalmol Vis Sci. 1995 Jan;36(1):41–51. [PubMed] [Google Scholar]
- Mietz H., Cassinotti P., Siegl G., Kirchhof B., Krieglstein G. K. Detection of herpes simplex virus after penetrating keratoplasty by polymerase chain reaction: correlation of clinical and laboratory findings. Graefes Arch Clin Exp Ophthalmol. 1995 Nov;233(11):714–716. doi: 10.1007/BF00164675. [DOI] [PubMed] [Google Scholar]
- Morris D. J., Cleator G. M., Klapper P. E., Cooper R. J., Biney E. O., Dennett C., Marcyniuk B., Tullo A. B. Detection of herpes simplex virus DNA in donor cornea culture medium by polymerase chain reaction. Br J Ophthalmol. 1996 Jul;80(7):654–657. doi: 10.1136/bjo.80.7.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Neufeld M. V., Steinemann T. L., Merin L. M., Stroop W. G., Brown M. F. Identification of a herpes simplex virus-induced dendrite in an eye-bank donor cornea. Cornea. 1999 Jul;18(4):489–492. doi: 10.1097/00003226-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin D., Sundmacher R., Wochnik G., Bablok B. Herpes simplex virus types 1 and 2 in ocular disease. Arch Ophthalmol. 1978 Jan;96(1):64–69. doi: 10.1001/archopht.1978.03910050028007. [DOI] [PubMed] [Google Scholar]
- O'Brien W. J., Tsao L. S., Taylor J. L. Tissue-specific accumulation of latency-associated transcripts in herpes virus-infected rabbits. Invest Ophthalmol Vis Sci. 1998 Sep;39(10):1847–1853. [PubMed] [Google Scholar]
- Power W. J., Hogan R. N., Hu S., Foster C. S. Primary varicella-zoster keratitis: diagnosis by polymerase chain reaction. Am J Ophthalmol. 1997 Feb;123(2):252–254. doi: 10.1016/s0002-9394(14)71043-8. [DOI] [PubMed] [Google Scholar]
- Remeijer L., Doornenbal P., Geerards A. J., Rijneveld W. A., Beekhuis W. H. Newly acquired herpes simplex virus keratitis after penetrating keratoplasty. Ophthalmology. 1997 Apr;104(4):648–652. doi: 10.1016/s0161-6420(97)30257-7. [DOI] [PubMed] [Google Scholar]
- Shimeld C., Whiteland J. L., Nicholls S. M., Grinfeld E., Easty D. L., Gao H., Hill T. J. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995 Aug;61(1):7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- Sterk C. C., Jager M. J., Swart-vd Berg M. Recurrent herpetic keratitis in penetrating keratoplasty. Doc Ophthalmol. 1995;90(1):29–33. doi: 10.1007/BF01203291. [DOI] [PubMed] [Google Scholar]
- Tambasco F. P., Cohen E. J., Nguyen L. H., Rapuano C. J., Laibson P. R. Oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Arch Ophthalmol. 1999 Apr;117(4):445–449. doi: 10.1001/archopht.117.4.445. [DOI] [PubMed] [Google Scholar]
- Tsurumi T., Maeno K., Nishiyama Y. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene. 1987;52(2-3):129–137. doi: 10.1016/0378-1119(87)90039-4. [DOI] [PubMed] [Google Scholar]
- Tullo A. B., Marcyniuk B., Bonshek R., Dennett C., Cleator G. M., Lewis A. G., Klapper P. E. Herpes virus in a corneal donor. Eye (Lond) 1990;4(Pt 5):766–767. doi: 10.1038/eye.1990.111. [DOI] [PubMed] [Google Scholar]
- Wilson S. E., Kaufman H. E. Graft failure after penetrating keratoplasty. Surv Ophthalmol. 1990 Mar-Apr;34(5):325–356. doi: 10.1016/0039-6257(90)90110-h. [DOI] [PubMed] [Google Scholar]
- Zheng X., Marquart M. E., Loustch J. M., Shah P., Sainz B., Ray A., O'Callaghan R. J., Kaufman H. E., Hill J. M. HSV-1 migration in latently infected and naive rabbits after penetrating keratoplasty. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2490–2497. [PubMed] [Google Scholar]
- de Boer J. H., Verhagen C., Bruinenberg M., Rothova A., de Jong P. T., Baarsma G. S., Van der Lelij A., Ooyman F. M., Bollemeijer J. G., Derhaag P. J. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996 Jun;121(6):650–658. doi: 10.1016/s0002-9394(14)70631-2. [DOI] [PubMed] [Google Scholar]