Abstract
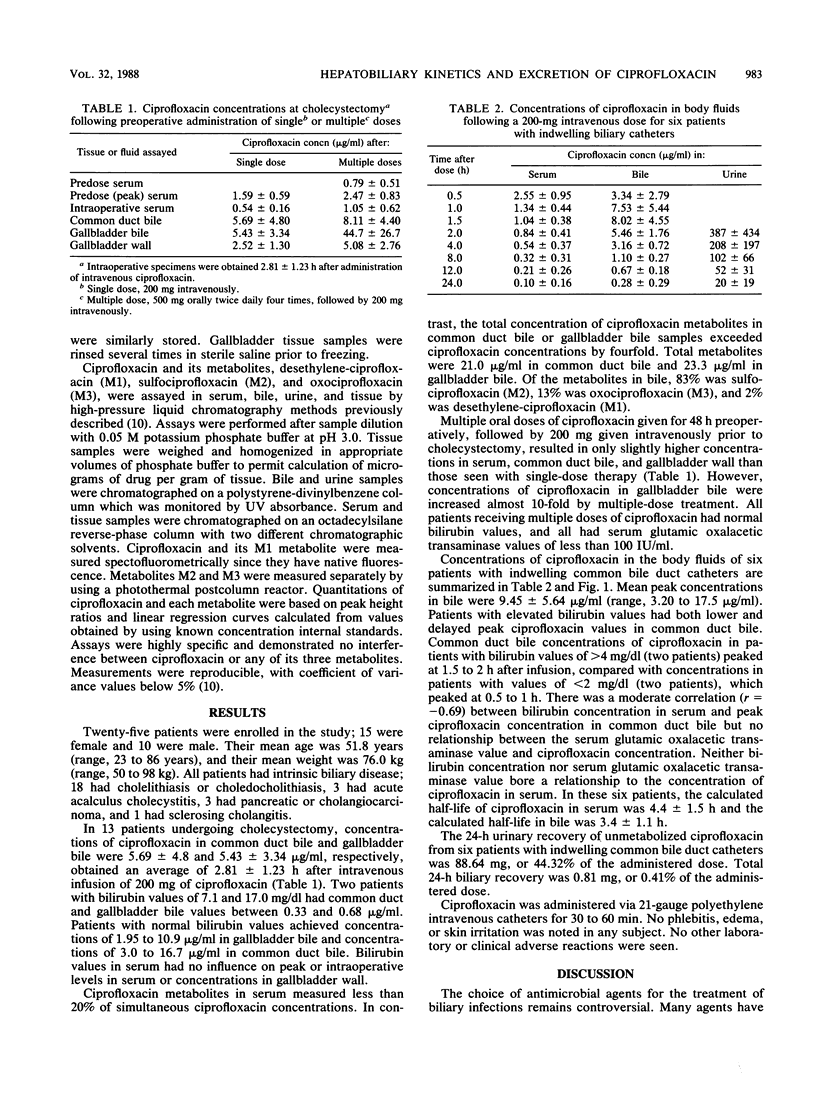
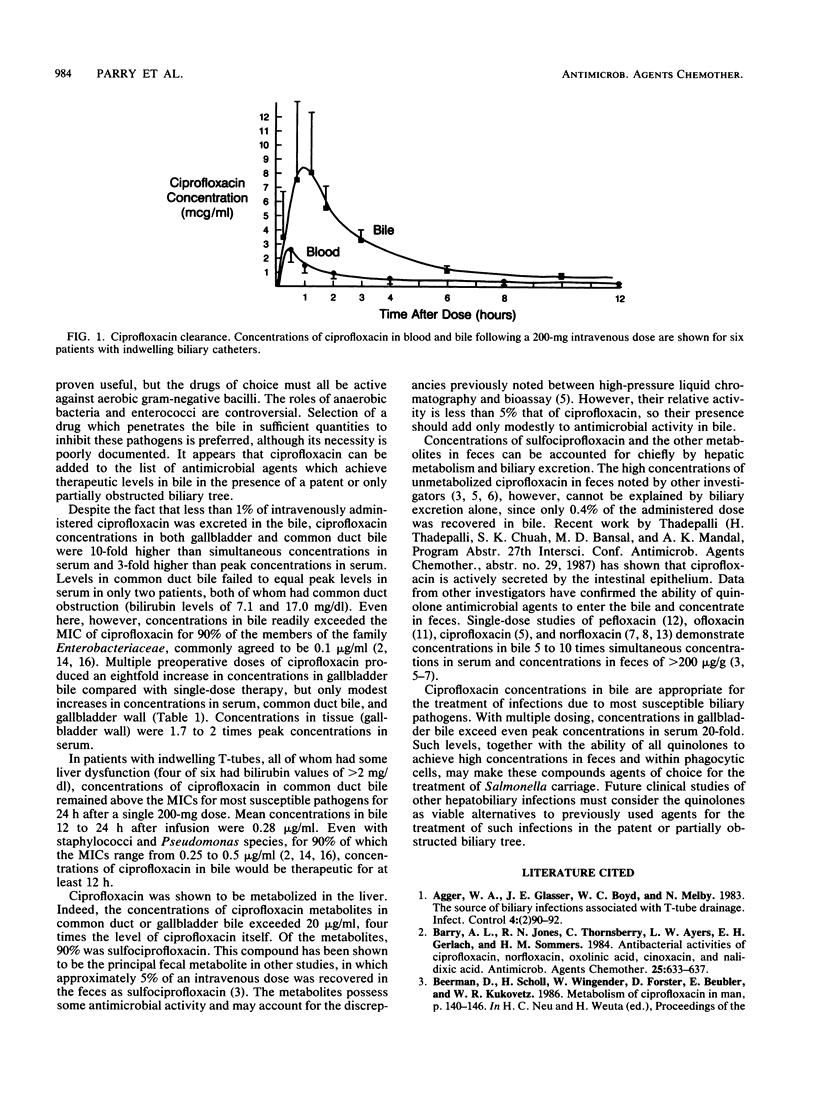
The biliary excretion and metabolism of ciprofloxacin was studied in 25 hospitalized patients: 19 undergoing routine cholecystectomy and 6 with indwelling biliary drainage catheters. An intravenous dose of 200 mg of ciprofloxacin given 2.5 to 3.0 h prior to cholecystectomy resulted in concentrations in common duct bile, gallbladder bile, and gallbladder wall of 5.69 +/- 4.8, 5.43 +/- 3.34, and 2.52 +/- 1.30 micrograms/g, respectively, all at least fourfold greater than simultaneous concentrations in serum. Ciprofloxacin concentrations in common duct bile exceeded peak concentrations in serum in all but two patients with common duct obstruction. Multiple preoperative doses of ciprofloxacin prior to cholecystectomy increased concentrations in gallbladder bile by eightfold. Six patients with indwelling biliary drainage catheters also received 200 mg of ciprofloxacin intravenously. Less than 1% of the administered dose was excreted in bile as unchanged ciprofloxacin, and there was extensive metabolism. However, peak ciprofloxacin concentrations of 2.83 +/- 0.76 micrograms/ml in serum produced peak concentrations of 10.69 +/- 5.30 micrograms/ml in bile within 1.5 h after infusion and maintained concentrations of at least 0.5 microgram/ml in common duct bile for over 12 h in all patients. It appears that ciprofloxacin concentrations in bile will exceed the MICs for most susceptible biliary pathogens for a period of at least 12 h after a 200-mg intravenous dose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agger W. A., Glasser J. E., Boyd W. C., Melby N. The source of biliary infections associated with T-tube drainage. Infect Control. 1983 Mar-Apr;4(2):90–92. doi: 10.1017/s0195941700057817. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boey J. H., Way L. W. Acute cholangitis. Ann Surg. 1980 Mar;191(3):264–270. doi: 10.1097/00000658-198003000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogard J. M., Jehl F., Monteil H., Adloff M., Blickle J. F., Levy P. Comparison of high-pressure liquid chromatography and microbiological assay for the determination of biliary elimination of ciprofloxacin in humans. Antimicrob Agents Chemother. 1985 Aug;28(2):311–314. doi: 10.1128/aac.28.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Franklin I., Grady D., Hamilton-Miller J. M., Iliffe A. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):757–761. doi: 10.1128/aac.26.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofsky R. D., duBouchet L., Landesman S. H. Recovery of norfloxacin in feces after administration of a single oral dose to human volunteers. Antimicrob Agents Chemother. 1984 Jul;26(1):110–111. doi: 10.1128/aac.26.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan M., Serour F., Gorea A., Levenberg A., Krispin M., Berger S. A. Concentration of norfloxacin in human gallbladder tissue and bile after single-dose oral administration. Antimicrob Agents Chemother. 1987 Feb;31(2):352–353. doi: 10.1128/aac.31.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keighley M. R., Drysdale R. B., Quoraishi A. H., Burdon D. W., Alexander-Willians J. Antibiotics in biliary disease: the relative importance of antibiotic concentrations in the bile and serum. Gut. 1976 Jul;17(7):495–500. doi: 10.1136/gut.17.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk J. P., Campoli-Richards D. M. Ofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1987 Apr;33(4):346–391. doi: 10.2165/00003495-198733040-00003. [DOI] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt H. A., Postier R. G., Cameron J. L. Biliary bacteria: significance and alterations after antibiotic therapy. Arch Surg. 1982 Apr;117(4):445–449. doi: 10.1001/archsurg.1982.01380280037008. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. Overview of preclinical studies with ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):2–11. [PubMed] [Google Scholar]
- Shimada K., Noro T., Inamatsu T., Urayama K., Adachi K. Bacteriology of acute obstructive suppurative cholangitis of the aged. J Clin Microbiol. 1981 Nov;14(5):522–526. doi: 10.1128/jcm.14.5.522-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


